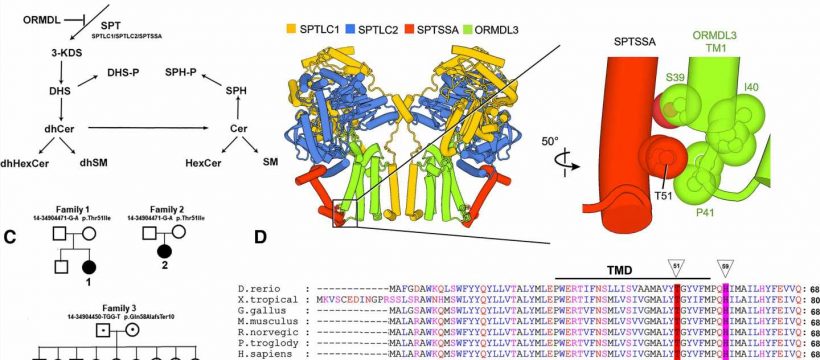
![SL biosynthesis and HSP-causing variants in the SPTSSA subunit of SPT. (A) SPT catalyzes the first and rate-limiting step of sphingolipid synthesis, the condensation of serine with an acyl-CoA (typically, palmitoyl-CoA) to generate the sphingoid base, 3-KDS, which is further modified to form the complex family of SLs. SPT is feedback inhibited by the ORMDL proteins. The dihydroceramide-based SLs [dihydroceramide (dhCer), dihydrohexosylceramide (dhHexCer) and dihydrosphingomyelin (dhSM)] do not usually accumulate to appreciable levels, but excessive SPT activity results in significant elevation of these SLs. (B) The SPT/ORMDL3 complex is a dimer of SPTLC1/SPTLC2/SPTSSA/ORMDL3 tetramers. The enlarged image (right) shows that Thr51 of SPTSSA is in close contact with the luminal end of the first (of four) transmembrane domain (TM1) of ORMDL3. (C) Pedigrees showing de novo occurrence of the heterozygous SPTSSA p.Thr51Ile variant in two families and the inherited p.Gln58AlafsTer10 variant in the third family. Filled symbols denote probands; dots in the center of the circle or square denote carriers. (D) Thr51 (indicated by triangle) of the evolutionarily conserved SPTSSA subunit is located at the luminal end of the single transmembrane domain (TMD). Genetic mutation linked to Hereditary Spastic Paraplegia](https://scx1.b-cdn.net/csz/news/800a/2023/genetic-mutation-linke.jpg)
A genetic mutation in the SPTSSA gene is identified as the cause of hereditary spastic paraplegia, a rare disease that causes progressive weakness, stiffness and spasticity in the lower extremities, according to a study published in Brain. The SPTSSA gene is responsible for stimulating serine palmitoyltransferase, or SPT, an enzyme with critical functions within the nervous system.
The researchers from the Uniformed Services University of the Health Sciences (USU) in collaboration with Massachusetts General Hospital (MGH), found that two of three non-related patients with a complex case of HSP had the same mutation in the SPTSSA gene. In both of those cases, this was a new mutation that their parents did not have. This mutation changes a single unit in the string of amino acids, which make up the SPTSSA protein.
In the third patient, a young man with a less severe case of HSP, the researchers found an inherited mutation in SPTSSA causing his SPTSSA protein to be shorter than normal. His asymptomatic parents and several unaffected siblings had one mutant and a normal copy of the SPTSSA gene, and this patient inherited the mutant copy of the SPTSSA gene from each parent.
The researchers determined that these disease-causing SPTSSA variants prevent the normal regulation of sphingolipid synthesis. SPTSSA is a subunit of an enzyme—SPT, or serine palmitoyltransferase. The SPT enzyme catalyzes the first step in producing sphingolipids—a diverse family of lipids with critical cellular functions that are highly enriched in the nervous system.
Mutations in other subunits of SPT are known to cause other neurological diseases, like HSAN1, the most common type of inherited peripheral neuropathy, and a juvenile-onset form of ALS. If regulation of SPT is working properly, when sphingolipid levels become too high, they bind to the SPT/ORMDL complex and inhibit SPT activity. However, the SPTSSA variants impair this regulation and lead to unrestrained SPT activity. This results in an overproduction of sphingolipids that leads to neurodegenerative disease.
Although only a small number of patients are affected—an estimated 20,000 people in the U.S. suffer from HSP—the impact on families is enormous. In addition to progressive limb spasticity, which characterizes “pure” HSP—where muscles stiffen or tighten due to a disruption between the brain and the spinal cord, patients with a complex form of the disease, including these SPTSSA patients, also display variable sensory and cognitive involvement.
So far, more than 80 genetic mutations have been associated with the various forms of HSP, but this is the first report that perturbed sphingolipid homeostasis causes this disease.
These findings bolster the growing body of evidence that mutations in the subunits of SPT can lead to different types of neurological disease. They also make it clear that disturbances in sphingolipid synthesis underlie multiple neurological diseases, and that correcting sphingolipid homeostasis could be used as a potential treatment strategy for these devastating diseases.
“We’re extremely excited by these findings, which are consistent with our earlier studies on juvenile ALS-causing variants,” said Dr. Teresa Dunn, co-lead author on the study and chair of USU’s Department of Biochemistry and Molecular Biology.
“Together, this study and our previous research will be critically important for understanding how perturbed regulation of sphingolipid levels lead to neurodegenerative disease and raise the possibility that other genes associated with HSP and ALS play a role in maintaining sphingolipid homeostasis. These studies also point to sphingolipids as potential biomarkers and suggest potential treatment strategies.”
This study is also important in providing clues as to what metabolic pathways are involved in many other diseases with similar clinical symptoms, Dunn added.
SPTSSA was first discovered by Dunn and coworkers a decade ago. Ongoing studies in Dunn’s lab have shown that SPTSSA plays a role in the feedback inhibition of SPT by proteins called the ORMDLs that regulate SPT function. As a result, when SPTSSA variants were discovered in patients, the team was well positioned to determine the mechanism of the disease, Dunn explained.
“This discovery of these gene defects ends the diagnostic odyssey for a number of families affected by progressive motor problems. Recognizing the importance of sphingolipid regulation during early brain development ushers in a new chapter in neurobiology,” said Dr. Florian Eichler, neurologist and director of the Leukodystrophy Service at MGH.
The study was spearheaded by USU’s Dunn and Massachusetts General Hospital’s Dr. Florian Eichler, a pediatric neurologist, in collaboration with the Undiagnosed Disease Network (UDN) and several other universities, including Duke University, Baylor University, and Hebrew University.
More information:
Siddharth Srivastava et al, SPTSSA variants alter sphingolipid synthesis and cause a complex hereditary spastic paraplegia, Brain (2023). DOI: 10.1093/brain/awac460
Journal information:
Brain
Source: Read Full Article