A team of experts from the University of Barcelona, the University of Zaragoza and Hospital Clínic de Barcelona has found a mechanism of recruitment for tumor-associated cells (cancer-associated fibroblasts or CAFs), which are essential to lung adenocarcinoma, the most frequent type of cancer. These cancer-associated cells contribute to all the phases of tumor development, including metastasis.
The study, published in the British Journal of Cancer, reveals there is a type of inhibitor drug that could be useful against the migratory advantage of these cancer-associated cells, and so could prevent their recruitment and therefore their contribution to the tumor development.
“The importance of these findings lies in the fact that lung adenocarcinoma represents 40% of the cases of lung cancer and it produces an early metastasis, which directly affects the patients’ chances of survival,” says lecturer Jordi Alcaraz, coordinator of the study and member of the Faculty of Medicine and Health Sciences of the UB and the Institute of Bioengineering of Catalonia (IBEC), Currently, the five-year survival rate for lung cancer that has not spread to other organs is more than 60%. However, when it spreads to other parts of the body, these chances are reduced to below 10%.
How does SMAD3 protein affect the tumor process?
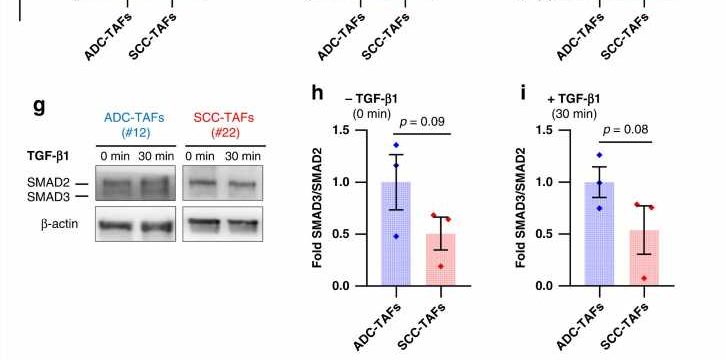
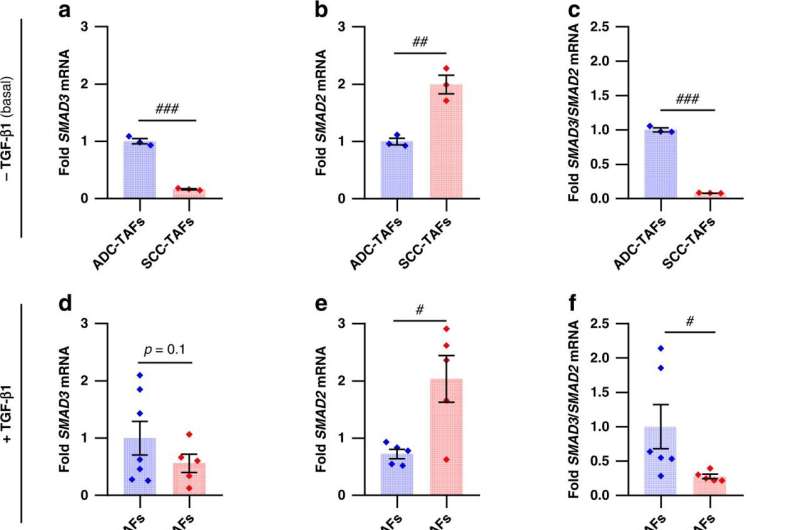
Alcaraz found in previous studies that the SMAD3 protein is selectively overactivated in patients with adenocarcinoma. Now, the new study, conducted together with the University of Zaragoza, analyzes the effects of the SMAD3 protein in the cancer-associated cell recruitment, and it analyzes its impact in the tumor dissemination and metastasis generation.
The team led by Professor José Manuel García Aznar, from the UZ University Research Institute on Engineering in Aragón (I3A), in which researchers Yago Juste Lanas and Carlos Borau take part, applied an innovative technology based on microfluidic devices with 3D collagen extracellular matrices to study the cancer-cell protrusions and cell migration in environments that simulate different stages of tumor development.
Cancer-associated cells showed a migratory advantage—faster and more directional movement—in an environment typical of the early stages of cancer. Also, the researchers observed in these cancer-associated cells a lower proliferative capacity, which involved the SMAD3 promigratory effect as an essential factor for the recruitment and accumulation of CAFs in adenocarcinoma.
As these cancer-associated cells contribute to all the phases of tumor development—including the dissemination—this finding could be decisive for understanding the early dissemination of adenocarcinoma to other organs. Additionally, this migratory advantage was removed by the inhibitor Trametinib, which is already approved to be used in other types of tumors.
According to Lanas, the first author of the study, the researchers “found that the adenocarcinoma tumor-associated cells have a high migratory capacity. This enables their recruitment to the tumor more easily, and it could favor an early metastasis formation, a process observed in patients but for which the causes are still unknown. In addition, inhibitors such as Trametinib, according to our results, could be effective against recruitment.”
In environments close to a more developed tumor, researchers have stated that adenocarcinoma-associated cells can establish closer interactions with tumor cells due to the reduction of the migratory capacity seen in this study.
“We continue to work to understand whether lung adenocarcinoma-associated cells can also promote the spread of these tumors by other mechanisms, with the ultimate aim of curbing their metastasis,” note the researchers.
More information:
Yago Juste-Lanas et al, 3D collagen migration patterns reveal a SMAD3-dependent and TGF-β1-independent mechanism of recruitment for tumour-associated fibroblasts in lung adenocarcinoma, British Journal of Cancer (2022). DOI: 10.1038/s41416-022-02093-x
Journal information:
British Journal of Cancer
Source: Read Full Article