A recent article published on the bioRxiv* preprint server reports that the coronavirus disease 2019 (COVID-19) NVX-CoV2373 vaccine triggers robust neutralization against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron subvariants.
Study: Novavax NVX-COV2373 triggers potent neutralization of Omicron sub-lineages. Image Credit: Studio Roux / Shutterstock.com
Background
The SARS-CoV-2 Omicron variant of concern (VOC) and its subvariants BA.4, BA.5, BA.2, and BA.2.12.1 possess numerous spike (S) mutations that are responsible for the high level of resistance observed against COVID-19 vaccine-induced neutralization, irrespective of the vaccine platform. Likewise, individuals with a history of prior COVID-19 also exhibit reduced neutralizing titers against several Omicron subvariants.
Nevertheless, booster vaccination has been shown to improve Omicron neutralizing capacity, particularly with messenger ribonucleic acid (mRNA) vaccines.
NVX-CoV2373 is a COVID-19 protein nanoparticle vaccine that has been particularly useful in nations with limited cold-chain requirements. The NVX-CoV2373 vaccine is listed in the World Health Organization (WHO) emergency use category for COVID-19 vaccines and has been granted usage authorization by the European Medicines Agency.
About the study
In the current study, researchers report SARS-CoV-2 neutralizing titers, including those against several Omicron sublineages, after double and triple vaccination with the NVX-CoV2373 vaccine. To this end, they evaluated how a third dose affected the ability of the NVX-CoV2373 vaccine recipients’ sera to neutralize SARS-CoV-2 and these variants.
The neutralization of the SARS-CoV-2 ancestral D614G, Omicron BA.4, BA.1, BA.5, and Beta strains by the sera of 29 and 48 NVX-CoV2373 vaccine recipients was assessed after completion of a two- and three-dose vaccine regimens, respectively. Omicron BA.4, BA.5, and BA.1 neutralization were also assessed after multiple doses of mRNA, adenoviral, and protein-based COVID-19 vaccines.
Study findings
Geometric mean titers (GMTs), which were measured 14 days after NVX-CoV2373 two-dose vaccination, peaked against the SARS-CoV-2 D614G variant with a GMT of 1,401. GMTs subsequently decreased to 34, 47, and 173 against Omicron BA.1, Omicron BA.4/BA.5, and Beta, respectively.
Notably, Omicron BA.4/BA.5 and BA.1 subvariants were impervious to neutralization with titers below the detection range for the assay by 72% following two NVX-CoV2373 vaccine doses.
Neutralizing antibody response with titers exceeding 1:200 against the Omicron BA.1 and Beta variants was noticeable one month after the third NVX-CoV2373 dose in 47 of 48 samples. In addition, neutralizing antibody titers against the SARS-CoV-2 D614G strain were increased to a GMT of 10,862.
Moreover, a third NVX-CoV2373 dose resulted in improved titers against Omicron BA.1, BA.4/BA.5, and Beta, with GMTs of 1,197, 582, and 1,733, respectively. Nevertheless, these titers were six- to eight times lower than those against D614G.
Following three NVX42 CoV2373 doses, all except one sample had Omicron subvariant titers higher than 50% protection levels.
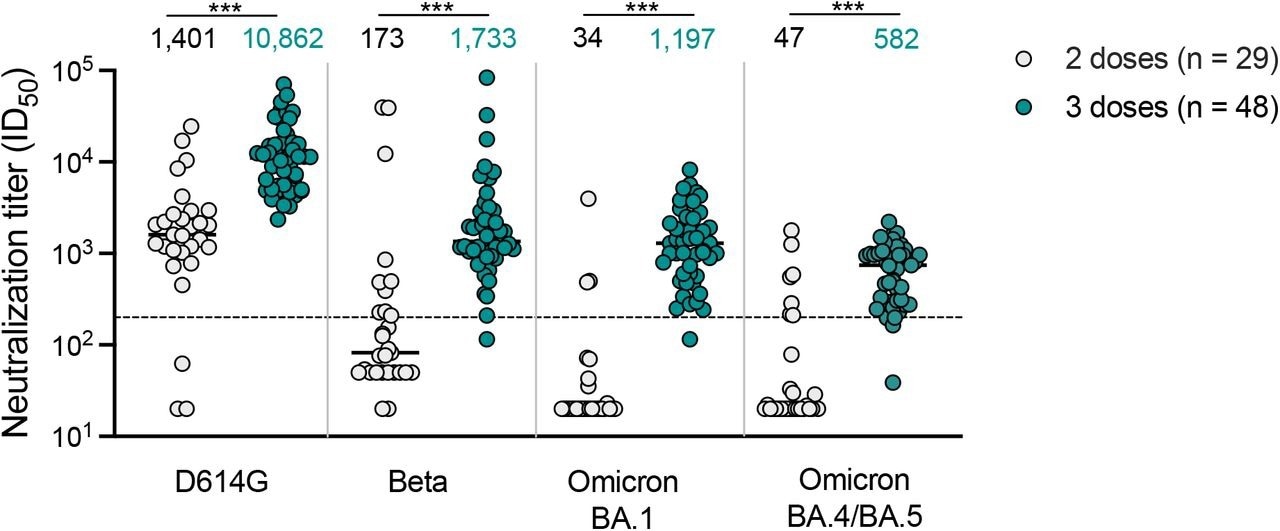 Neutralization of SARS-CoV-2 variants by NVX-CoV2373 vaccinee plasma. Neutralization of ancestral D164G, Beta, Omicron BA.1 and Omicron BA.4/BA.5 pseudoviruses by NVX-CoV2373 vaccinee plasma following 2 (grey) or 3 (teal) doses. Geometric mean titers (GMT) for each virus are shown above the individual points, and percent of specimens where no neutralization was observed (red) is indicated in the pie charts. Number of vaccinee specimens tested are indicated and p values were calculated using the Mann-Whitney t-test for non-parametric data with p < 0,001 for D614G, Beta, Omicron BA.1 and Omicron BA.4/BA.5. Dashed line indicates the neutralization level at 20.2% of the mean convalescent level (ID50 = 200), which provides an estimated 50% protection against detectable SARS-CoV-2 infection per the analysis by Khoury et al15. Samples were used at a starting dilution of 1 in 20 (limit of detection) with a seven 3-fold dilutions to create a titration series.
Neutralization of SARS-CoV-2 variants by NVX-CoV2373 vaccinee plasma. Neutralization of ancestral D164G, Beta, Omicron BA.1 and Omicron BA.4/BA.5 pseudoviruses by NVX-CoV2373 vaccinee plasma following 2 (grey) or 3 (teal) doses. Geometric mean titers (GMT) for each virus are shown above the individual points, and percent of specimens where no neutralization was observed (red) is indicated in the pie charts. Number of vaccinee specimens tested are indicated and p values were calculated using the Mann-Whitney t-test for non-parametric data with p < 0,001 for D614G, Beta, Omicron BA.1 and Omicron BA.4/BA.5. Dashed line indicates the neutralization level at 20.2% of the mean convalescent level (ID50 = 200), which provides an estimated 50% protection against detectable SARS-CoV-2 infection per the analysis by Khoury et al15. Samples were used at a starting dilution of 1 in 20 (limit of detection) with a seven 3-fold dilutions to create a titration series.
Two doses of the Johnson & Johnson AD26.COV2.S vaccine induced 14- and 10-fold lower GMT responses against the Omicron BA.1 variant as compared to three NVX-CoV2373 and Pfizer-BioNTech mRNA BNT162b2 vaccine doses, respectively. Likewise, the AD26.COV2.S vaccine stimulated 11- and 12-fold lower GMTs targeting BA.4/BA.5 than three NVX-CoV2373 and BNT162b2 vaccine doses, respectively.
Only 13-38% of AD26.COV2.S samples neutralized Omicron BA.4/BA.5 and BA.1 at titers higher than 200. This was comparable to the majority of plasma samples exhibiting this level of protection from three NVX-CoV2373 and BNT162b2 vaccine dose recipients.
Given the reduced GMTs for all three boosted vaccine regimens against BA.4/BA.5, 91% of NVX-CoV2373 and 83%, of BNT162b2 samples relative to 13% of AD26.COV.S samples neutralized BA.5/BA.4 at titers higher than 1:200 thresholds. The GMTs targeting BA.4/BA.5 of the NVX-CoV2373 three-dose vaccinated plasma was similar to BNT162b2, whereas the BA.1 neutralization titers were superior in the NVX-COV2373 cohort.
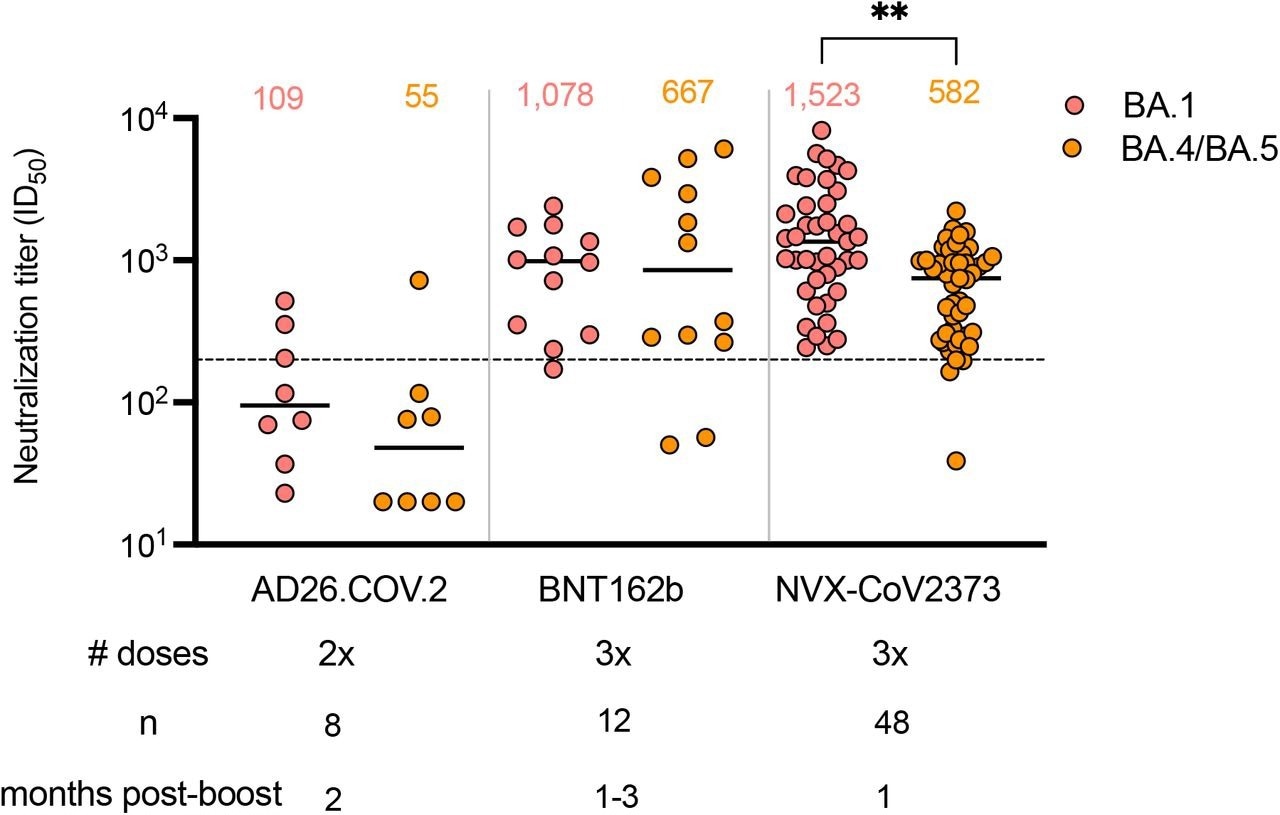 Neutralization of Omicron BA.1 and BA.4/BA.5 by boosted vaccinee plasma. Neutralization of Omicron BA.1 and BA.4/BA.5 by vaccinee plasma following 2 doses of the AD26.COV2S or 3 doses of the BNT162b2 or NVX-CoV2373 vaccines. Number of doses, number of samples and date of sample collection after boost for each group are indicated. Geometric mean titers (GMT) for each virus are shown above the individual points, P values were calculated using two-way ANOVA with p < 0,001 for AD26CoV2.S versus NXV-CoV2373 and p = 0,0011 for NVX-CoV2373 BA.1 versus BA.4/BA.5). Dashed line indicates the neutralization level at 20,2% of the mean convalescent level (ID50 = 200), which provides an estimated 50% protection against detectable SARS-CoV-2 infection per the analysis by Khoury et al.
Neutralization of Omicron BA.1 and BA.4/BA.5 by boosted vaccinee plasma. Neutralization of Omicron BA.1 and BA.4/BA.5 by vaccinee plasma following 2 doses of the AD26.COV2S or 3 doses of the BNT162b2 or NVX-CoV2373 vaccines. Number of doses, number of samples and date of sample collection after boost for each group are indicated. Geometric mean titers (GMT) for each virus are shown above the individual points, P values were calculated using two-way ANOVA with p < 0,001 for AD26CoV2.S versus NXV-CoV2373 and p = 0,0011 for NVX-CoV2373 BA.1 versus BA.4/BA.5). Dashed line indicates the neutralization level at 20,2% of the mean convalescent level (ID50 = 200), which provides an estimated 50% protection against detectable SARS-CoV-2 infection per the analysis by Khoury et al.
Conclusions
The findings from the current study demonstrate that three doses of the COVID-19 NVX-CoV2373 vaccine significantly improved neutralization of the SARS-CoV-2 Omicron BA.4/BA.5 and BA.1 subvariants with responses that were comparable to those achieved with three mRNA vaccine doses.
Notably, enhanced binding antibody levels were detected six months after two NVX-CoV2373 doses. Thus, future studies should evaluate NVXCoV2373 neutralization at later time points, as the longevity of different vaccine platforms can vary.
Since the SARS-CoV-2 Omicron BA.4 variant has become the dominant circulating strain in multiple regions, the study findings emphasize the potential use of NVX-CoV2373 vaccination as a booster dose, particularly in regions with limited resources.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Bhiman, J. N., Richardson, S. I., Lambson, B. E., et al. (2022). Novavax NVX-COV2373 triggers potent neutralization of Omicron sub-lineages. bioRxiv. doi:10.1101/2022.07.14.500148. https://www.biorxiv.org/content/10.1101/2022.07.14.500148v1.
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Antibody, Assay, Cold, Coronavirus, Coronavirus Disease COVID-19, covid-19, Nanoparticle, Omicron, Protein, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Titration, Vaccine, Virus

Written by
Shanet Susan Alex
Shanet Susan Alex, a medical writer, based in Kerala, India, is a Doctor of Pharmacy graduate from Kerala University of Health Sciences. Her academic background is in clinical pharmacy and research, and she is passionate about medical writing. Shanet has published papers in the International Journal of Medical Science and Current Research (IJMSCR), the International Journal of Pharmacy (IJP), and the International Journal of Medical Science and Applied Research (IJMSAR). Apart from work, she enjoys listening to music and watching movies.
Source: Read Full Article
