Scientists attacking the problem of high miscarriage rates have long wondered if there is a way to tell whether an egg cell will successfully develop into an embryo and grow or if there is a marker indicating when it is destined to fail.
Two Rutgers-led research teams have found strong clues in two separate studies using both human and mouse data that will allow them to begin to answer “yes” to both questions.
Reporting in Nature Communications, one team found that mouse egg cells that form an unusual cap-like structure before being fertilized are more likely to be viable, attach to the womb and grow than egg cells without the structure.
“These are important findings because, as many people seek [in vitro fertilization] for family building, success rates are low,” said Karen Schindler, a professor in the Department of Genetics in the Rutgers School of Arts and Sciences (SAS) and senior author of the paper. “Understanding the basic mechanisms of what makes a high-quality egg and embryo are essential for improving clinical success rates.”
In the second study, published in The American Journal of Human Genetics, the Rutgers-led team identified a gene that when mutated causes an abnormal number of chromosomes in mouse eggs—a leading cause of early miscarriage and in vitro fertilization (IVF) failure.
“We are seeking to understand the genetic roots of female infertility,” said Jinchuan Xing, a professor in the Department of Genetics in SAS and senior author of the paper. “In this case, the method we developed for identifying genetic risk can be applied by many researchers for further inquiry.”
Infertility, defined as the inability to conceive after one year or longer of unprotected sex, is a common problem. In the United States, among females ages 15 to 49 with no previous births, about 1 in 5 or 19% are unable to get pregnant after one year of trying, according to the U.S. Centers for Disease Control and Prevention. Also, about 1 in 4 or about 26% of women in this group have difficulty getting pregnant or carrying a pregnancy to term, a condition known as impaired fecundity, the CDC said.
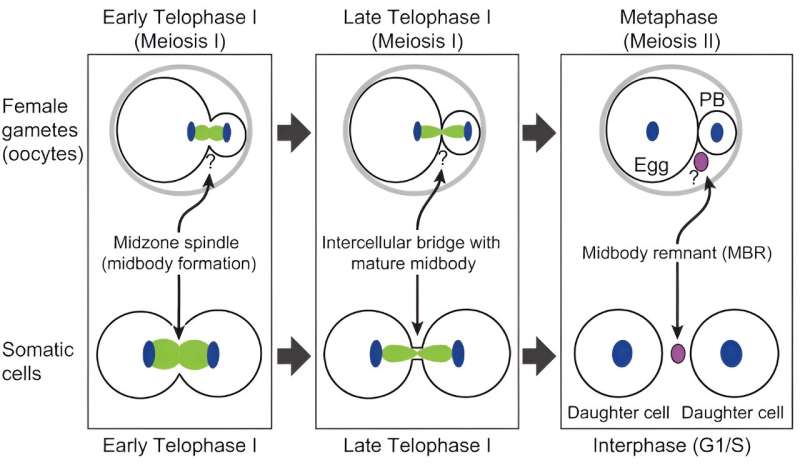
Schindler, Xing and their teams want to understand how some women produce highly viable eggs and why the process that produces eggs is so error prone. In Schindler’s study, the team zeroed in on one of the last steps of the egg production process. Schindler said the team was inspired by work on cancer cells by a colleague, Ahna Skop, a geneticist at the University of Wisconsin who is an author on the paper. Skop discovered that the region that forms between dividing cells contains essential materials such as RNAs and proteins.
Because an embryo relies on these essential materials to develop, Schindler wondered whether a mechanism with life-protecting proteins could also be produced when an egg cell divides into two daughter cells.
Unlike other cell types, egg cells that divide into two cells form them unequally. One, the egg, receives most of the vital material, such as genetic information and the structures that produce proteins, while the second, known as the polar body, receives little and eventually withers away and dies.
Using a microscope that produces high-resolution images of living cells, the Schindler team found that egg cells also have a region between the dividing cells that is enriched in essential materials. In this analysis, they discovered a new cap-like structure that forms between the cells. In egg cells that are successfully fertilized and grow into embryos, the caps form a protective barrier that prevent the essential materials from escaping into the adjoining polar body cell. In egg cells where the cap was disrupted, embryos were not viable.
“The cap is the boundary between the egg that will become fertilized by sperm and the non-functional polar body,” Schindler said. “Without this cap, essential materials can leak into the polar body and the egg is less likely to become an embryo.”
In the second paper, Xing and his team analyzed a pool of data collected by IVF clinics during genetic testing of embryos for an abnormal number of chromosomes before implantation. Xing said the data gathered in this collection method, which employs an inexpensive DNA sequencing technology, hasn’t been regarded as useful for in-depth searches of genetic patterns.
Although this low-coverage whole-genome sequencing method produces a fraction of the data from each genetic sample and relies on computational methods to fill in the missing information, Xing’s team was able to detect a gene mutation common to egg failure. When tested in mice, the mutation causes mistakes in the number of chromosomes divided between the egg and the polar body.
“The findings and the method used have broad implications, not only for clinicians and patients investigating emerging causes of IVF failure, but in providing the world with a new way to do genetic studies using low-coverage sequencing data,” Xing said.
More information:
Gyu Ik Jung et al, An oocyte meiotic midbody cap is required for developmental competence in mice, Nature Communications (2023). DOI: 10.1038/s41467-023-43288-x
Siqi Sun et al, Identifying risk variants for embryo aneuploidy using ultra-low coverage whole-genome sequencing from preimplantation genetic testing, The American Journal of Human Genetics (2023). DOI: 10.1016/j.ajhg.2023.11.002
Journal information:
American Journal of Human Genetics
,
Nature Communications
Source: Read Full Article
