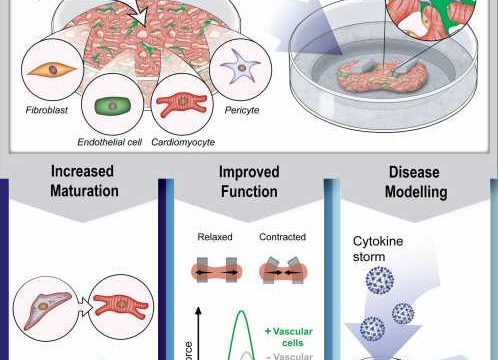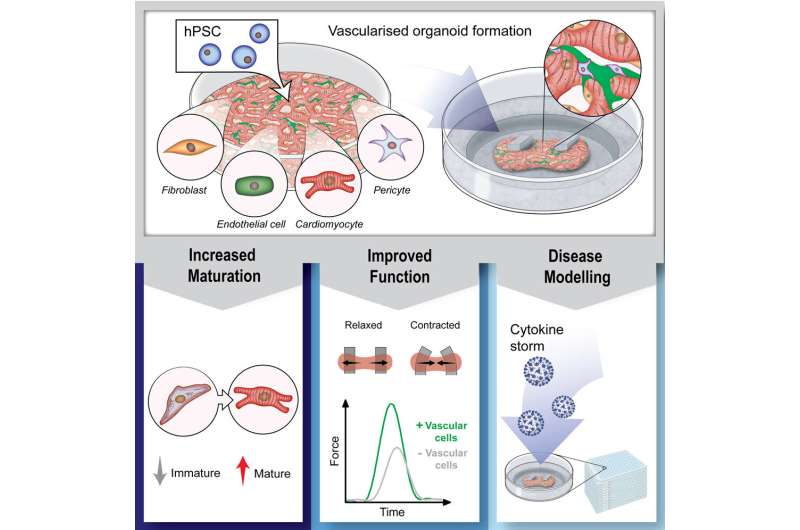
An Australian research team led by QIMR Berghofer has succeeded in introducing a vascular system into tiny living and beating model human heart muscles, an achievement which it’s hoped will accelerate progress toward the ultimate goal of repairing damage from heart disease.
The findings, published in the journal Cell Reports, have also for the first time revealed the central role the vascular system plays in causing inflammation-driven injury of the heart muscle, which is important for several diseases that can cause heart injury including COVID-19.
The new vascularized tiny heart muscles, or organoids, more closely mimic the human heart and will allow much more accurate testing of new drugs to treat disease and inflammation, and take scientists a step closer to the holy grail of repairing heart tissue.
Lead researcher Professor James Hudson, who heads QIMR Berghofer’s Cardiac Bioengineering Research Group, said vascularizing the tiny hearts is a game changer for their work.
“We only know a fraction about the biology underpinning the heart so we’re constantly trying to improve our cardiac organoids to simulate the heart’s complex cellular interactions and tissue composition.
“Each organoid is only about the size of a chia seed, measuring just 1.5 millimeters across, but inside are 50,000 cells representing the different cell types that make up the heart,” Professor Hudson said.
Organoids are grown from human pluripotent stem cells which can be generated using “reprogramming” of skin or blood cells. Until now, the model hearts included a range of cell types including the cells that hold the tissue together and the cells that make them beat, but researchers had not been able to add the critical vascular cells.
“Incorporating the vascular cells for the first time in our mini heart muscles is very significant because we found they had a key role in the biology of the tissues. Vascular cells made the organoids function better and beat strongly. This has really opened up our ability to better understand the heart and accurately model disease,” Professor Hudson said.
The team is focused on finding therapeutics to repair different types of heart damage. One of those is inflammation, which is the body’s reaction to insults such as metabolic disease or COVID-19, causing the heart to stiffen so it fails to fully relax and fill with enough blood.
“When we simulated inflammation in our mini heart muscles, we found the vascular cells played a central role. We only saw the stiffening in the tissues that had the vascular cells. The cells sensed what was happening and changed their behavior, and we identified that the cells release a factor called endothelin that mediates the stiffening. We can now target this mechanism to see if we can control it with new therapeutics,” Professor Hudson said.
Cardiovascular disease is the leading cause of death in Australia claiming the lives of around 18,000 people each year. It is a major burden on the health system costing around $12 billion annually. The trend is predicted to worsen due to an aging population and lifestyle factors.
“Heart disease is devastating for the patient and their loved ones. And it is a huge burden on the economy. Finding new treatments is crucial to addressing this.
“For one type of heart failure that we work on, preserved ejection fraction (HFpEF), there is only one therapeutic available, so we urgently need to identify new drugs to improve patient outcomes and reduce the burden of heart disease.
“That’s where our new system of producing vascularised cardiac organoids will really give us an advantage because we’ll be able to progress the search for new treatments much more quickly,” Professor Hudson said.
Publication of the research will assist researchers around the world to replicate the vascularised organoids and boost the global effort to tackle heart disease. The method also has broader implications that could help researchers in other fields creating organoids such as kidneys and brains.
More information:
Holly K. Voges et al, Vascular cells improve functionality of human cardiac organoids, Cell Reports (2023). DOI: 10.1016/j.celrep.2023.112322
Journal information:
Cell Reports
Source: Read Full Article