The ENSEMBLE clinical trial, which aimed to test the safety and efficacy of the Johnson & Johnson vaccine (Ad26.COV2.S) against moderate to severe coronavirus disease 2019 (COVID-19), was conducted in the United States, South Africa, and selected countries of Latin America including Mexico, Argentina, Brazil, Chile, Colombia, and Peru.
This double-blind, randomized, and placebo-controlled trial was designed to evaluate the impact of a single dose of the vaccine. The primary evaluation suggested a vaccine efficacy of 66.1% which, in turn, led to its authorization for emergency use in more than a hundred countries across the globe.
Study: Immune Correlates Analysis of a Single Ad26.COV2.S Dose in the ENSEMBLE COVID-19 Vaccine Efficacy Clinical Trial. Image Credit: Aha-Soft / Shutterstock.com
What is the need for COVID-19 vaccine correlate of protection?
Finding an immunological correlate of vaccine-induced protection has remained a concern for many reasons. One major reason is to fill in the shortage of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines across the globe, particularly in low and middle-income countries. Although several COVID-19 vaccines have been licensed to date, due to the long periods involved in vaccine production, the world urgently needs more vaccines to be licensed to satisfy the need.
Many of these COVID-19 vaccines are not able to undergo Phase III clinical trials due to shortages in the number of sick individuals, which means the clinical trial will take much longer to complete. For these candidates, having an absolute correlate of protection will provide validation on the basis of certain immune thresholds, thus circumventing delay issues and would therefore boost global vaccine availability.
The immune correlates will also aid in evaluating the consistency of vaccine quality over time, the immune status of individuals after vaccination, validating/licensing of heterologous vaccines in further studies, and assist in predicting the durability of protection.
About the study
In a recent study published on the preprint server medRxiv*, the ENSEMBLE data was used to evaluate three immune markers as correlates of protection following a single dose of Ad26.COV2.S vaccine. While all three antibody markers including anti-spike (S) immunoglobulin G (IgG) binding antibody, anti-receptor binding domain (RBD) IgG antibody, and pseudovirus neutralizing antibody (PsVNA) titers demonstrated correlation, the neutralizing antibody titers represented the strongest evidence of correlation.
The comparative analysis found the results to be consistent across two more vaccine trials including the COVE trial of the mRNA-1273 (Moderna) vaccine and the COV002-UK trial of the AZD1222 (AstraZeneca) vaccine, which also demonstrated the neutralizing antibody titers (nAb) as the correlate of vaccine protection.
Immune correlates were assessed in a subset of the ENSEMBLE Phase III cohort on day 29 post-Ad26.COV2.S vaccine administration. The subset involved healthy, as well as vaccinated individuals, who suffered a breakthrough infection.
Of the subset, 826 individuals received the vaccine and 90 individuals received the placebo, with 50.4% of the study participants older than 60 years old and 51.7% with associated comorbidities. Vaccine breakthrough infections were reported in 92 cases.
The individuals who did not suffer SARS-CoV-2 infection up to the end of the correlates study period, which was defined as 54 days post day 29, were defined as non-cases (baseline seronegative individuals). During the time of the study, ‘Reference’ (Wuhan-Hu-1 strain with D614G mutation) and ‘Other’ (strain that varies from reference but still constitute the same lineage) strains were prevalent in the U.S. Beta lineages were prevalent in South Africa, whereas Reference, Zeta, and Other strains were prevalent in Latin America.
Non-cases had higher antibody titers than the vaccine breakthrough cases
With respect to the three antibody markers amongst the non-cases, a positive S-IgG and RBD-IgG response was seen in 85.3% and 81.2% of non-cases, respectively, and 56.4% had quantifiable inhibitory dilution 50% (ID50) nAb titers. The response rate for each antibody marker was of lower magnitude in breakthrough cases than in the non-cases, with the largest difference recorded for ID50 nAb. The geometric mean values of all three markers were also lower in breakthrough cases than in non-cases at day 29.
The trends for a cumulative incidence of COVID-19 showed that each antibody marker at day 29 correlated inversely with the risk of breakthrough infection. That is, the risk of breakthrough infection decreased with increasing antibody titers.
The observed hazard ratio for S-IgG, RBD-IgG, and nAb-ID50 were 0.75, 0.61, and 0.41, respectively. The point estimates and high confidence intervals indicated nAb-ID50 as the strongest correlate.
Vaccine efficacy also increased with rising antibody marker levels and the rise was the highest for the nAb-ID50 titers. The efficacy was observed to be 60% at low unmeasurable nAb-ID50 levels, which increased to 89% at 96.3 IU50/ml.
Vaccine efficacy increased with higher D29 antibody marker levels, with results supporting the importance of achieving quantifiable antibodies, as negative binding antibody response and nonquantifiable neutralization corresponded to marginal vaccine efficacy of about 50%.”
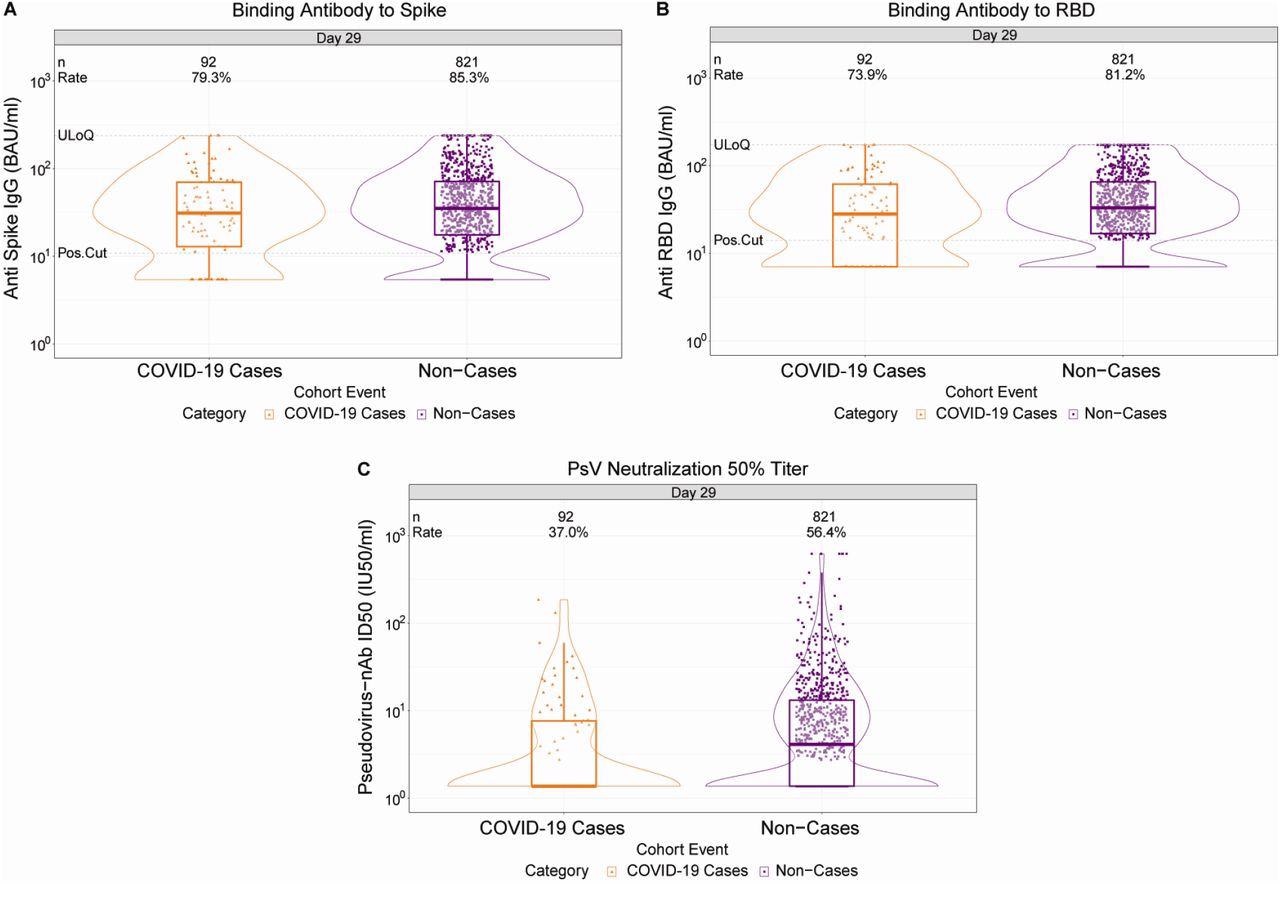
D29 antibody marker level by COVID-19 outcome status. (A) Anti-spike IgG concentration, (B) anti-receptor binding domain (RBD) IgG concentration, and (C) pseudovirus (PsV) neutralization ID50 titer. Data points are from baseline SARS-CoV-2 seronegative perprotocol vaccine recipients in the set. The violin plots contain interior box plots with upper and lower horizontal edges the 25th and 75th percentiles of antibody level and middle line the 50th percentile, and vertical bars the distance from the 25th (or 75th) percentile of antibody level and the minimum (or maximum) antibody level within the 25th (or 75th) percentile of antibody level minus (or plus) 1.5 times the interquartile range. At both sides of the box, a rotated probability density curve estimated by a kernel density estimator with a default Gaussian kernel is plotted. Positive response rates were computed with inverse probability of sampling weighting. Pos.Cut, Positivity cut-off. Positive response for spike IgG was defined by IgG > 10.8424 BAU/ml and for RBD IgG was defined by IgG > 14.0858 BAU/ml. ULoQ, upper limit of quantitation. ULoQ = 238.1165 BAU/ml for spike IgG and 172.5755 BAU/ml for RBD IgG. LLoQ, lower limit of quantitation. Positive response for ID50 was defined by value > LLoQ (2.7426 IU50/ml). ULoQ = 619.3052 IU50/ml for ID50. Cases are baseline SARS-CoV-2 seronegative per-protocol vaccine recipients with the primary COVID-19 endpoint (moderate to severe-critical COVID-19 with onset both ≥ 1 day post D29 and ≥ 28 days post-vaccination) up to 54 days post D29 but no later than January 22, 2021.
Vaccine efficacy
Similar trends of increasing vaccine efficacy with day 29 nAb-ID50 titers were seen for all geographic regions under comparison. However, the vaccine efficacy curve from the U.S. trended upwards as compared to the curve from South Africa, which trended upwards in comparison to the curve for Latin America.
The estimated relationship of ID50 titer with vaccine efficacy, that appeared to differ between the US, Latin America and South Africa, could be explained by the greater match of the vaccine strain to the reference strain (which predominated in the US) compared to the different strains that circulated in Latin America and South Africa.”
The researchers also compared vaccine efficacy between three double-blinded and placebo-controlled COVID-19 vaccine efficacy clinical trials (ENSEMBLE, COVE, and COV002 trials) with different COVID-19 vaccine formulations as well as different dosing schedules. To this end, each trial showed the same trend of rising vaccine efficacy with increasing nAb-ID50 titer.
Future directions
Further studies are needed to assess the ability of the day 29 nAb-ID50 marker in predicting vaccine efficacy against other SARS-CoV-2 strains like the Delta and Omicron variants, which were not encountered during the current study/trial period. In addition, future studies need to verify the validity of correlates over extended follow-up periods.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Fong, Y., McDermott, A. B., Benkeser, D., et al. (2022) Immune Correlates Analysis of a Single Ad26.COV2.S Dose in the ENSEMBLE COVID-19 Vaccine Efficacy Clinical Trial. medRxiv. doi:10.1101/2022.04.06.22272763. https://www.medrxiv.org/content/10.1101/2022.04.06.22272763v1.
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Antibodies, Antibody, Clinical Trial, Coronavirus, Coronavirus Disease COVID-19, Efficacy, Immunoglobulin, Mutation, Omicron, Placebo, Pseudovirus, Receptor, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, Vaccine

Written by
Namita Mitra
After earning a bachelor’s degree in Veterinary Sciences and Animal Health (BVSc) in 2013, Namita went on to pursue a Master of Veterinary Microbiology from GADVASU, India. Her Master’s research on the molecular and histopathological diagnosis of avian oncogenic viruses in poultry brought her two national awards. In 2013, she was conferred a doctoral degree in Animal Biotechnology that concluded with her research findings on expression profiling of apoptosis-associated genes in canine mammary tumors. Right after her graduation, Namita worked as Assistant Professor of Animal Biotechnology and taught the courses of Animal Cell Culture, Animal Genetic Engineering, and Molecular Immunology.
Source: Read Full Article
