Immunotherapy—drug treatment that stimulates the immune system to attack tumors—works well against some types of cancer, but it has shown mixed success against lung cancer.
A new study from MIT helps to shed light on why the immune system mounts such a lackluster response to lung cancer, even after treatment with immunotherapy drugs. In a study of mice, the researchers found that bacteria naturally found in the lungs help to create an environment that suppresses T-cell activation in the lymph nodes near the lungs.
The researchers did not find that kind of immune-suppressive environment in lymph nodes near tumors growing near the skin of mice. They hope that their findings could help lead to the development of new ways to rev up the immune response to lung tumors.
MIT graduate student Maria Zagorulya is the lead author of the paper, which appears today in the journal Immunity.
“There is a functional difference between the T-cell responses that are mounted in the different lymph nodes. We’re hoping to identify a way to counteract that suppressive response, so that we can reactivate the lung-tumor-targeting T cells,” says Stefani Spranger, the Howard S. and Linda B. Stern Career Development Assistant Professor of Biology, a member of MIT’s Koch Institute for Integrative Cancer Research, and the senior author of the new study.
Failure to attack
For many years, scientists have known that cancer cells can send out immunosuppressive signals, which leads to a phenomenon known as T-cell exhaustion. The goal of cancer immunotherapy is to rejuvenate those T cells so they can begin attacking tumors again.
One type of drug commonly used for immunotherapy involves checkpoint inhibitors, which remove the brakes on exhausted T cells and help reactivate them. This approach has worked well with cancers such as melanoma, but not as well with lung cancer.
Spranger’s recent work has offered one possible explanation for this: She found that some T cells stop working even before they reach a tumor, because of a failure to become activated early in their development. In a 2021 paper, she identified populations of dysfunctional T cells that can be distinguished from normal T cells by a pattern of gene expression that prevents them from attacking cancer cells when they enter a tumor.
“Despite the fact that these T cells are proliferating, and they’re infiltrating the tumor, they were never licensed to kill,” Spranger says.
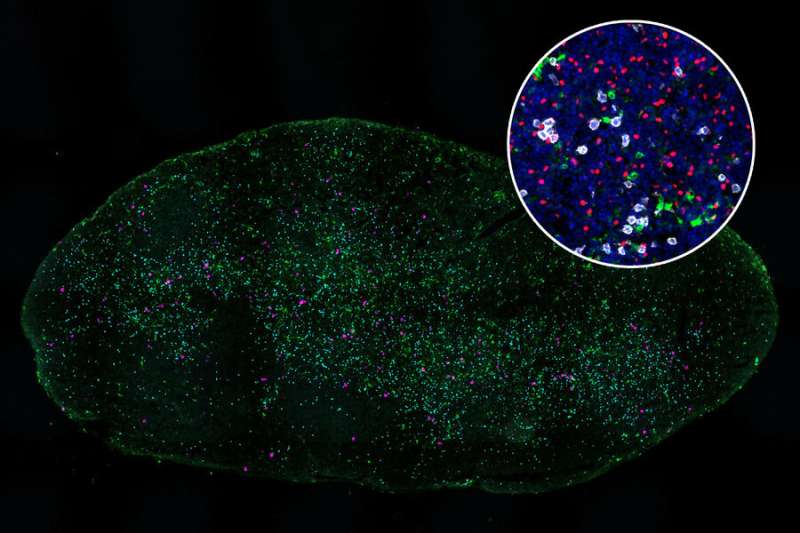
In the new study, her team delved further into this activation failure, which occurs in the lymph nodes, which filter fluids that drain from nearby tissues. The lymph nodes are where “killer T cells” encounter dendritic cells, which present antigens (tumor proteins) and help to activate the T cells.
To explore why some killer T cells fail to be properly activated, Spranger’s team studied mice that had tumors implanted either in the lungs or in the flank. All of the tumors were genetically identical.
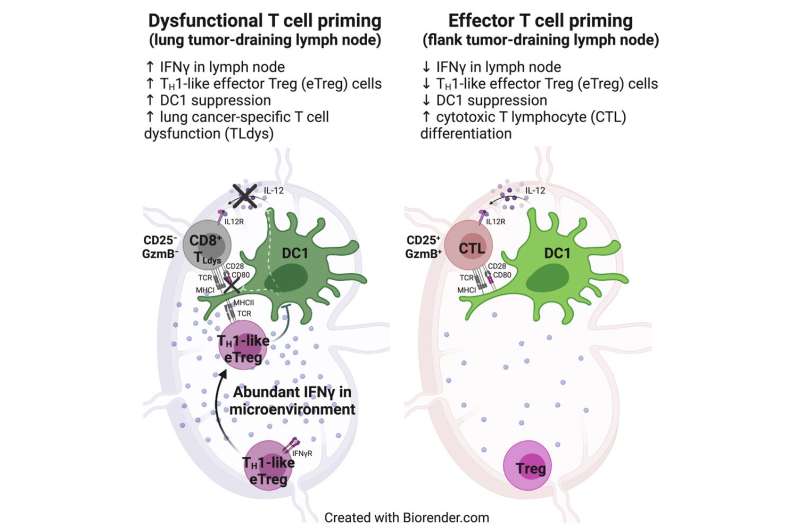
The researchers found that T cells in lymph nodes that drain from the lung tumors did encounter dendritic cells and recognize the tumor antigens displayed by those cells. However, these T cells failed to become fully activated, as a result of inhibition by another population of T cells called regulatory T cells.
These regulatory T cells became strongly activated in lymph nodes that drain from the lungs, but not in lymph nodes near tumors located in the flank, the researchers found. Regulatory T cells are normally responsible for making sure that the immune system doesn’t attack the body’s own cells. However, the researchers found that these T cells also interfere with dendritic cells’ ability to activate killer T cells that target lung tumors.
The researchers also discovered how these regulatory T cells suppress dendritic cells: by removing stimulatory proteins from the surface of dendritic cells, which prevents them from being able to turn on killer-T-cell activity.
Microbial influence
Further studies revealed that the activation of regulatory T cells is driven by high levels of interferon gamma in the lymph nodes that drain from the lungs. This signaling molecule is produced in response to the presence of commensal bacterial—bacteria that normally live in the lungs without causing infection.
The researchers have not yet identified the types of bacteria that induce this response or the cells that produce the interferon gamma, but they showed that when they treated mice with an antibody that blocks interferon gamma, they could restore killer T cells’ activity.
Interferon gamma has a variety of effects on immune signaling, and blocking it can dampen the overall immune response against a tumor, so using it to stimulate killer T cells would not be a good strategy to use in patients, Spranger says. Her lab is now exploring other ways to help stimulate the killer T cell response, such as inhibiting the regulatory T cells that suppress the killer-T-cell response or blocking the signals from the commensal bacteria, once the researchers identify them.
More information:
Maria Zagorulya et al, Tissue-specific abundance of interferon-gamma drives regulatory T cells to restrain DC1-mediated priming of cytotoxic T cells against lung cancer, Immunity (2023). DOI: 10.1016/j.immuni.2023.01.010
Journal information:
Immunity
Source: Read Full Article
