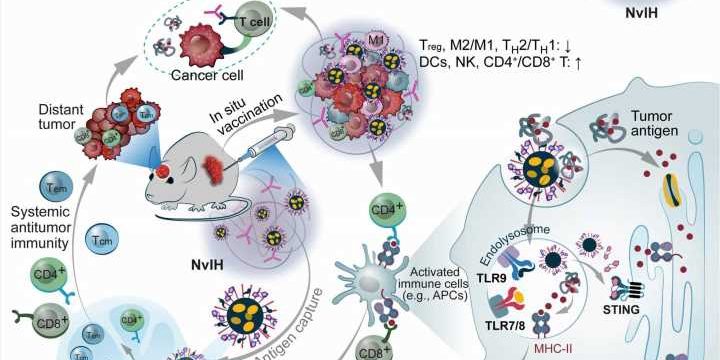
The present variants of cancer immunotherapy such as immune checkpoint blockades only benefit a small subset of patients due to its immunosuppressive tumor microenvironment. The capacity to vaccinate tumors and reduce immunosuppression in the microenvironment is a significant method to improve cancer immunotherapy.
In a new report now published in Science Advances, Furong Cheng and a team of scientists in translational medicine and pharmaceuticals in China and the U.S. presented single-dose injectable, nanovaccines and immune checkpoint blockade in a hydrogel to conduct robust immunotherapy of large tumors and abbreviated the invention as NvIH.
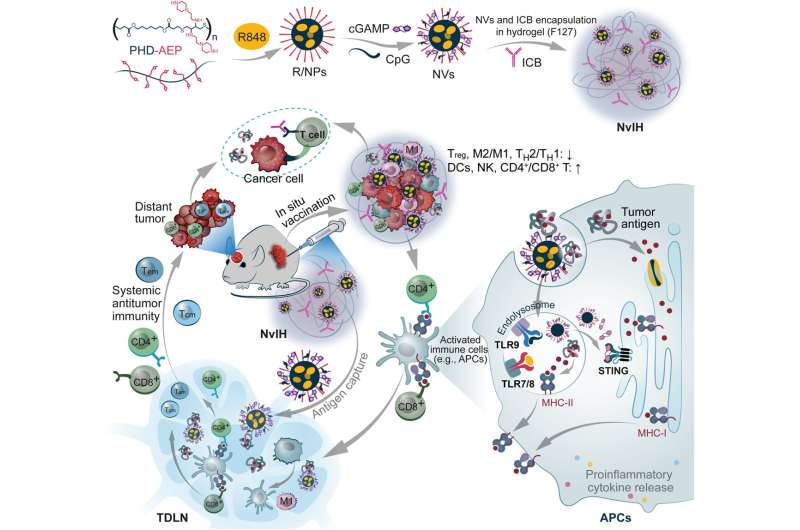
The cancer biologists and bioengineers developed the drug as a thermoresponsive hydrogel co-encapsulated with immune checkpoint blockade antibodies and polymeric nanoparticles loaded with immunostimulatory agonists and stimulators of interferon genes. They vaccinated the tumor environment and noted how the vaccine underwent rapid sol-to-gel transformation to prolong its retention in the area, sustain the release of tumor therapeutics and reduce systemic inflammation.
Cheng and colleagues administered the compound as a single-dose to reduce multitier immunosuppression of the tumor microenvironment, elicit potent immunity memory in the region with an abscopal impact (simultaneously impact both local and distant tumors) on the immune system, with additional impact on distant orthotopic glioblastoma. The injectable nanovaccine (NvIH) holds great potential for tumor immunotherapy.
Cancer immunotherapy
Cancer immunotherapy has a significant impact on many types of cancers, although existing methods only benefit a small subset of patients due to the immunosuppressive tumor microenvironment and insufficient filtration of anti-tumor T-cells. Many research approaches address these challenges by developing cancer vaccines, chemotherapy, radiotherapy and oncolytic virotherapy.
In this work, the team developed nanovaccines for simultaneous antigen cross-presentation, alongside multipronged pattern recognition receptor antitumor immunostimulation. Macroscale hydrogel and nanocarriers are biomaterials that can retain chemical and biological therapeutics to reduce off-target adverse side-effects. These results underscore the potential of NvIH for safe and robust immunotherapy of local and distant tumors.
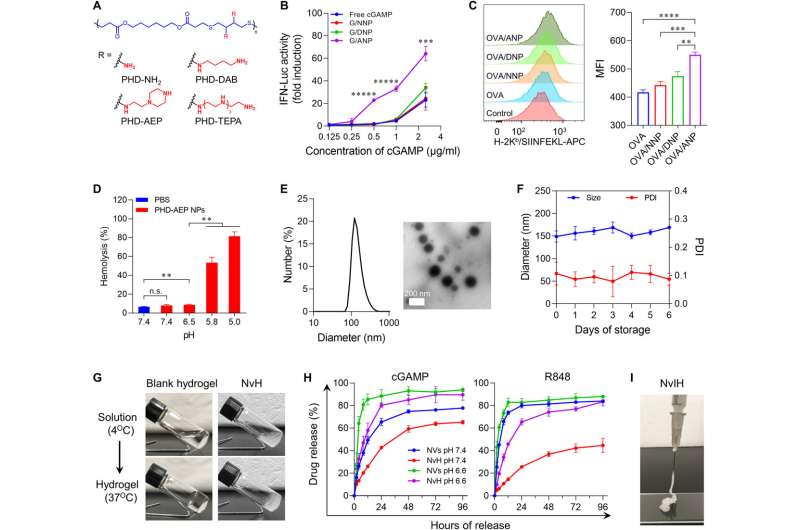
Bioengineering and characterizing the immunotherapy vaccine
The scientists added the immunostimulant-loaded nanovaccines within the hydrogel to regulate prolonged tissue retention of immunostimulants. Cheng and colleagues hypothesized that adding the immunostimulant-loaded nanovaccines in hydrogel can mediate their regulated release and prolong tissue retention of immunostimulants.
They synthesized these immunostimulant-loaded nanovaccines by designing cationic polymers to form nanoparticles with a hydrophobic core. They engineered polymeric nanocarriers with minimal toxicity and high immunostimulant loading and synthesized a series of cationic polymers via thiol-Michael addition polymerization and substitution reactions.
During the experiments, the team synthesized poly (β-thioester esters) and confirmed their consistency by using nuclear magnetic resonance and gel permeation chromatography. The scientists assessed the intracellular delivery of nanoparticles as immunostimulants.
Ideally, tumor vaccination can remodel immune milieu in the tumor microenvironment and elicit anti-tumor T-cell responses. The team facilitated prolonged release of immunostimulants by using a thermo-sensitive Pluronic hydrogel to prepare the constructs. The syringe injectable hydrogel enabled surgery-free minimally invasive syringe injection accompanied with fast gelation at physiological temperatures to accomplish prolonged retention in the tumor microenvironment alongside sustained release of drugs from the nanovaccine’s hydrogel reservoir.
Robust antigen-presenting cell (APC) activation by the hydrogel vaccine
The research team expected the co-delivery of immunostimulants to elicit potent anti-tumor immunostimulation. They first monitored the immunostimulant co-delivery efficiency by using flow cytometry and confocal microscopy. The team then studied the capacity of the hydrogel-based vaccine to activate antigen-presenting cells.
The outcomes showed enhanced pro-inflammatory responses and dendritic cell maturation, altogether with the ability of nanovaccines to capture antigens and produce robust antitumor T-cell responses. The scientists then investigated the retention rate of immunostimulants in the tumor microenvironment while monitoring potent anti-tumor immunity via in situ tumor vaccination with a single-dose of the nanovaccine hydrogel.
Antitumor modulation and immunotherapy of large tumors using single-dose vaccination
The research team studied the therapeutic impact of the nanovaccine/hydrogel in a mouse model subcutaneously inoculated with large, poorly immunogenic tumors. The outcomes showed negligible tumor growth inhibition with a control vaccine, while in contrast, the single-dose nanovaccine/hydrogel decreased tumor growth rate, while increasing mouse survival. The outcomes highlighted the dose-dependency of the therapeutic agent.
The researchers explored the vaccine with and without immune checkpoint inhibitor inclusion for immunotherapy of large, poorly immunogenic melanoma. The two variants showed robust immunotherapeutic efficiency of large tumors with proactive antitumor immunity and lasting immune memory.
Cheng and colleagues further studied the capacity to overcome cancer metastasis, and thereby prevent cancer patient death. They accomplished this by investigating systemic antitumor immunity for the therapeutic intervention of distant tumors inaccessible for in situ vaccination and administer effective immunotherapy with abscopal effects. They studied this with a mouse model to understand single-dose nanovaccination induced systemic antitumor immunity to treat primary and distant tumors.
The results showed great safety potential of the nanovaccine. The robust single-dose nanovaccine presented immunotherapy for primary vaccinated tumors, and for distant orthotopic glioblastomas that mimic human brain metastases to elicit systemic antitumor immune memory and prevent tumor relapse.
Outlook: Scope for effective nanovaccines for cancer therapy with robust immune memory to prevent relapse
In this way, Furong Cheng and the team investigated the dynamics of immunotherapy to treat a growing number of cancers. They bioengineered injectable nanoparticle-integrated hydrogel composites to develop the nanovaccines alongside immune checkpoint blockade within a hydrogel (NvIH) that contained triple immunostimulants to facilitate prolonged tumor retention via the regulated release of immunotherapeutics.
The scientists imparted robust immunotherapy of large local and distant tumors with abscopal effects across a variety of tumor models. These nanovaccines hold great potential for combined immunotherapy strategies to target a range of cancer types.
More information:
Furong Cheng et al, Single-dose injectable nanovaccine-in-hydrogel for robust immunotherapy of large tumors with abscopal effect, Science Advances (2023). DOI: 10.1126/sciadv.ade6257
Antoni Ribas et al, Cancer immunotherapy using checkpoint blockade, Science (2018). DOI: 10.1126/science.aar4060
Journal information:
Science Advances
,
Science
Source: Read Full Article