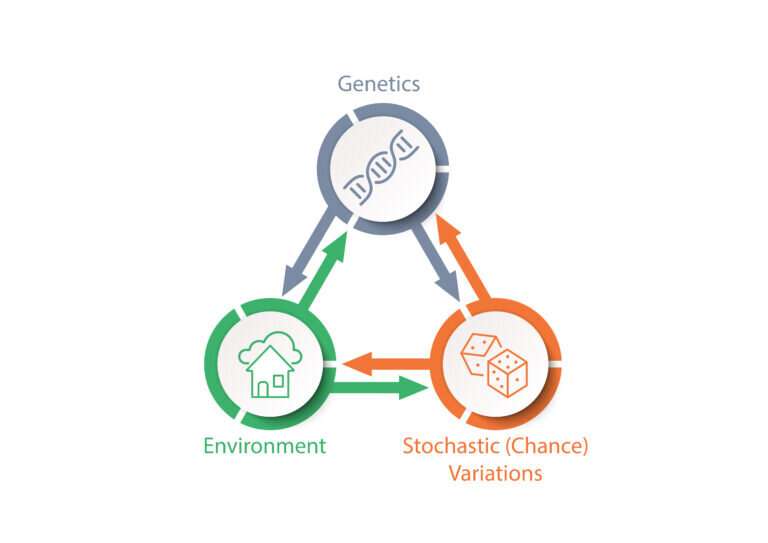
A new model of aging takes into account not only genetics and environmental exposures but also the tiny changes that randomly arise at the cellular level.
University Professor Caleb Finch introduced the “Tripartite Phenotype of Aging” as a new conceptual model that addresses why lifespan varies so much, even among human identical twins who share the same genes. Only about 10 to 35 percent of longevity can be traced to genes inherited from our parents, Finch mentioned.
Finch authored the paper introducing the model with one of his former graduate students, Amin Haghani, who received his Ph.D. in the Biology of Aging from the USC Leonard Davis School in 2020 and is now a postdoctoral researcher at UCLA. In the article, they propose that the limited heritability of aging patterns and longevity in humans is an outcome of gene-environment interactions, together with stochastic, or chance, variations in the body’s cells. These random changes can include cellular changes that happen during development, molecular damage that occurs later in life, and more.
“We wanted to introduce a conceptual map and some new terminology that will motivate a more comprehensive understanding of what the limitations of genetic determinants in aging are, how important it is to consider the genetic variance in relationship to the environment, and include this new domain of stochastic variations, which is very well recognized by different fields,” said Finch, who holds the ARCO/William F. Kieschnick Chair in the Neurobiology of Aging at the USC Leonard Davis School. “It hasn’t really been put in a formal context in which the complete package can be discussed, and that’s what I hope our article achieves.”
Expanding on the exposome
The new model is a natural extension of the idea of the exposome, which was first proposed by cancer epidemiologist Christopher Paul Wild in 2005 to draw attention to the need for more data on lifetime exposure to environmental carcinogens. The exposome concept illustrates how external factors, ranging from air pollution and socioeconomic status to individual diet and exercise patterns, interact with endogenous, or internal, factors such as the body’s microbiome and fat deposits.
The exposome is now a mainstream model, eclipsing previous characterizations of environmental factors as affecting risk “one by one.” Finch has previously expanded on the exposome concept with the introduction of the Alzheimer’s disease exposome. The gero-exposome now considers how genes and the environment interact over the lifespan to shape how we age.
The new model illustrates that cell-by-cell variations in gene expression, variations arising during development, random mutations, and epigenetic changes—turning genes “off” or “on”—should be explicitly considered apart from traditional genetic or environmental research regarding aging, Finch said. More detailed study into these chance processes has been enabled by cutting-edge research techniques, including the study of gene transcription within single cells as well as ChIP-sequencing, which can illustrate how individual proteins interact with DNA.
Effects of happenstance on health
In the paper, Finch and Haghani discussed several examples of how risks of age-related disease are poorly predicted by DNA alone but are heavily influenced by environmental exposures as well as the time and duration of the exposure, including during development or over the course of decades.
One well-known example of a gene that is associated with increased Alzheimer’s risk is ApoE-4; however, having the ApoE-4 gene doesn’t definitively mean someone will get Alzheimer’s. Studies in both mice and humans revealed that ApoE-4 and clusters of related genes interact with exposures such as air pollution or cigarette smoke to influence risk, and Alzheimer’s patients also show differences in their epigenetics as compared to individuals without the disease.
He added that the idea of environmental exposure can stretch farther than many people expect. Disease exposure earlier in life can affect health risks later in life—and across generations.
“The environment that we’re exposed to goes back to our grandmothers because the egg we came from was in our mother’s ovaries at the time of her birth,” he explained. “So that means, in my case, because my grandmother was born in 1878, I might very well carry some traces of the 19th century environment, which included much greater exposure to infectious disease because there were no antibiotics.”
Finch said that he hopes the more comprehensive model on how genes, environment, and random variations over time interact to influence aging prompt a new discussion of what the rapidly developing field of precision medicine needs to take into account to promote healthy aging.
Source: Read Full Article
