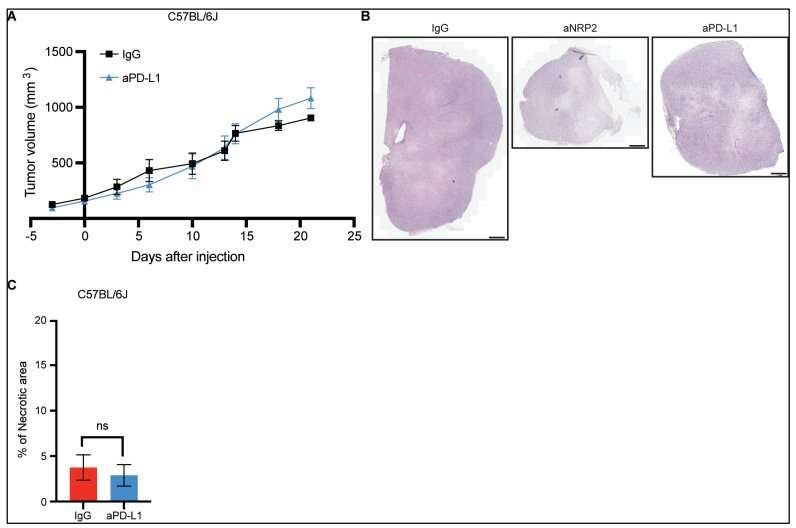
Therapeutic antibodies that cause aggressive tumors to become susceptible to treatment are being studied with the hope of one day changing the fate of patients who either relapse soon after cancer therapy or whose stubborn tumors repel potent medications rendering the drugs useless.
Although immunotherapies and chemotherapies improve outcomes for cancer patients, many cancers don’t respond well to treatment or thwart the drugs altogether. This is especially the case for aggressive prostate cancers, which are insensitive to immunotherapies.
A collaborative team of researchers throughout the United States, led by Chan School of Medicine scientists at the University of Massachusetts in Worchester, developed an antibody strategy that sensitizes prostate tumors to immunotherapy. The approach was tested in a mouse model using a monoclonal antibody dubbed aNRP2-28. Similar monoclonal antibodies, with the designation aNRP2-10, engineered for human cells, blocked the interaction between the growth factor VEGF (vascular endothelial growth factor) and its receptor neuropilin-2, thus sensitizing cells for treatment via immunotherapy.
Scientists found that by jamming the interaction between VEGF and neuropilin-2, they were able to render aggressive prostate cancers susceptible to treatment. This allowed use of the form of immunotherapy known as an immune checkpoint inhibitor. It’s critical, team members say, that the programmed cell death ligand, PD-L1, a protein found in abundance on prostate cancer cells be inhibited, which in turn, allows T cells to attack the cancer.
Writing in Science Translational Medicine, researchers report that prostate cancer cells in the laboratory were coaxed into submission and successfully treated. A second study in the same issue tackled triple negative breast cancer using the same monoclonal antibody strategy in cell lines, organoids and mouse models. Scientists demonstrated that this aggressive tumor type, which is notorious for its capacity to repel chemotherapy, can also be coaxed into submission and successfully treated with the form of chemotherapy that it otherwise would repel.
The challenge now, is determining whether these promising laboratory findings will prove equally successful in human clinical trials.
Unlike other solid tumors, prostate cancers do not respond particularly well to immune checkpoint inhibitors, such as the PD-L1 blockade, reports the lead the author of the research, Mengdie Wang of the Chan School of Medicine’s departments of molecular, cell and cancer biology.
“Prostate cancers are largely unresponsive to immune checkpoint inhibitors, and there is strong evidence that programmed death-ligand 1—PD-L1—expression itself must be inhibited to activate antitumor immunity,” Wang asserted. “We report that neuropilin-2, which functions as a vascular endothelial growth factor receptor on tumor cells, is an attractive target to activate antitumor immunity in prostate cancer because VEGF-NRP2 signaling sustains PD-L1 expression.”
Immune checkpoint inhibitor treatment is an innovative strategy that relies on the checkpoint blockade—blocking checkpoint proteins from binding with their partner proteins—preventing an “off” signal from being released. Checkpoint inhibitor treatments don’t kill tumor cells. Instead, the checkpoint inhibitor engages the immune system to recognize and attack malignancies, allowing T cells to kill the cancer.
There are several immune checkpoint inhibitors available to oncologists for cancer treatment. They include Keytruda, Yervoy, Opdivo and Tecentriq. Among the benefits offered by an immune checkpoint inhibitor treatment strategy is increased survival time and highly potent activity against cancer cells—when the cancer is responsive to the treatment.
To coax prostate cancer into responsiveness to this form of immunotherapy, Wang and colleagues engineered antibodies to suppress PD-L1 expression and tested the antibodies in humanized mouse models of prostate cancer and in patient-derived organoids with prostate cancer. The team found that the antibodies reduced PD-L1 expression in mice and in patient-derived organoids.
By suppressing PD-L1 expression this way, scientists found they could boost the infiltration of T cells into cancers and potentiate antitumor responses in mice with prostate tumors. They also observed unusually high activity among genes that encode PD-L1 and neuropilin-2 in samples from patients, suggesting that neuropilin-2 is a viable treatment target for the antibodies in humans.
Using monoclonal antibodies isn’t just for prostate cancers. In a second study, Zhiwen Xu of aTyr Pharma, Inc., a biotherapeutics company in San Diego, tested the efficacy of the monoclonal antibodies in cell lines, organoids, and mouse models of triple-negative breast cancer. Xu was also a member of Wang’s team, which tested the antibodies in the prostate cancer research.
When Xu and colleagues exposed mouse models for triple negative breast cancer to the antibodies, these proteins had potent effects against the tumor-seeding of cancer stem cells and prevented an epithelial-to-mesenchymal transition, a cell transformation linked to the progression of cancer.
The antibodies also sensitized cancerous tumors to the chemotherapy drug cisplatin by causing the cancer stem cells to differentiate into a more drug-sensitive form, leading to less metastasis in the rodents. “Based on the proposed mechanism of action, addition of [the antibodies] may lead to a deeper and prolonged clinical response in [triple-negative breast cancer] patients,” Xu concluded.
More information:
Mengdie Wang et al, Therapeutic blocking of VEGF binding to neuropilin-2 diminishes PD-L1 expression to activate antitumor immunity in prostate cancer, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.ade5855
Zhiwen Xu et al, Inhibition of VEGF binding to neuropilin-2 enhances chemosensitivity and inhibits metastasis in triple-negative breast cancer, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.adf1128
Journal information:
Science Translational Medicine
Source: Read Full Article