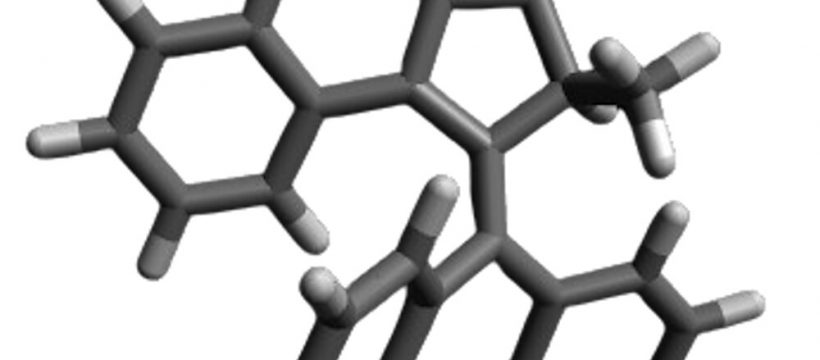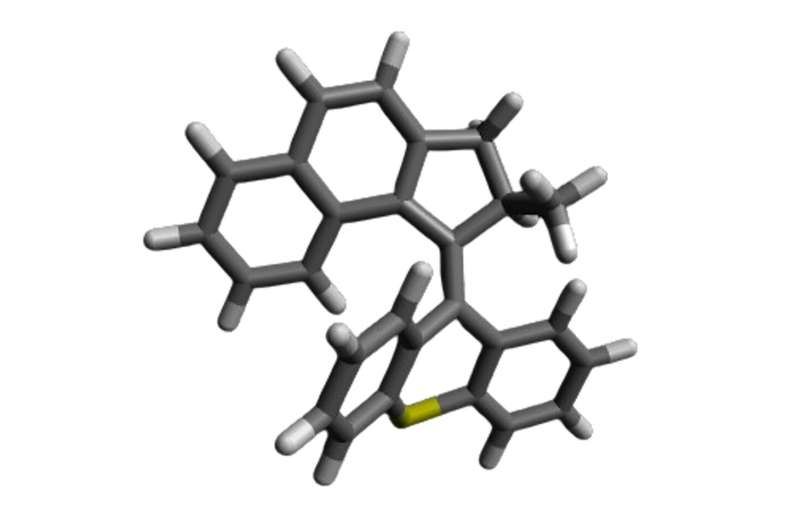
Fungi are present on the skin of around 70% of the population, without causing harm or benefit. Some fungal infections, like athlete’s foot, are minor. Others, like Candida albicans, can be deadly—especially for individuals with weakened immune systems.
Fungal infections are on the rise because of an aging population and an increased prevalence of chronic diseases. At the same time, fungi are becoming more resistant to treatment. As a result, fungal infections could soon become a serious public health threat.
In 2022, the World Health Organization released its first-ever “Fungal Priority Pathogen List,” calling for improved surveillance, public health interventions and the development of new antifungal drugs.
We are an interdisciplinary team of chemists and biologists charting a new path to tackle drug-resistant infections. We are using tiny nanoscale drills that combat harmful pathogens at the molecular level. As the traditional antimicrobial research pipeline struggles, our approach has the potential to rejuvenate the fight against these stubborn infections.
Molecular machines as alternative antifungals
While doctors urgently need new antifungal drugs, developing them is challenging. First, it is difficult to develop drugs that selectively kill fungi without harming human cells because of their many similarities.
Second, fungi can rapidly develop resistance to multiple antifungal drugs at once when medications are misused or overused. As such, developing antifungal drugs is much less rewarding for drug companies than developing medications for chronic conditions like diabetes and hypertension that require long-term use.
One solution to this problem could lie in a Nobel Prize-winning technology: molecular machines.
Molecular machines are synthetic compounds that rapidly rotate their components at about 3 million times per second when exposed to light. Doctors can use a light-tipped probe to activate these molecular machines to treat internal infections, or a lamp for skin infections. The light starts the machines spinning, and that rotational motion pushes them to drill through and puncture the cell’s membranes and organelles, which results in cell death.
Our group first used this technology to kill cancer cells in 2017. To target the right cells, molecular machines can be linked to specific peptides that bind only to the desired cells, allowing, for instance, the targeting of specific cancer types. Since then, we have used these molecules to kill bacteria, destroy tissue and stimulate muscle contraction. These properties make molecular machines an enticing candidate technology to address the growing fungal threat.
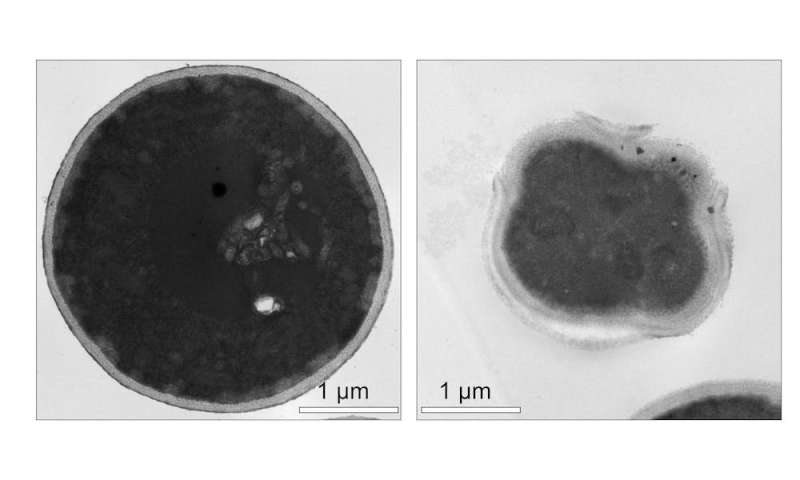
Testing antifungal molecular machines
Researchers first tested the ability of light-activated molecular machines to kill fungi in Candida albicans. This yeastlike fungus can cause life-threatening infections in immunocompromised people. Compared with conventional drugs, molecular machines killed C. albicans much faster.
Subsequent studies found that molecular machines could also kill other fungi, including molds like Aspergillus fumigatus and species of dermatophytes, the types of fungi that cause skin, scalp and nail infections. Molecular machines even eliminated fungal biofilms, which are slimy, antimicrobial-resistant communities of microorganisms that stick together on surfaces and commonly cause medical device-associated infections.
Unlike conventional antifungals, which target the fungal cell membrane or cell wall, molecular machines localize to the fungal mitochondria. Often referred to as the “powerhouses of the cell,” mitochondria produce energy to power other cellular activities. When activated with visible light, molecular machines destroy the fungal mitochondria. Once the fungal cell’s mitochondria stop working, the cell loses its energy supply and dies.
At the same time, molecular machines also disrupt the tiny pumps that remove antifungal agents from the cell, thus preventing the cell from fighting back. Because these molecular machines act by a mechanical instead of a chemical mechanism, fungi are unlikely to develop defenses against this treatment.
In lab experiments, combining light-activated molecular machines with conventional antifungal drugs also reduced the amount of fungi in C. albicans-infected worms and in pig nails infected with Trichophyton rubrum, the most common cause of athlete’s foot.
New frontiers for fighting fungal infections
These results suggest that combining molecular machines with conventional antifungals can improve existing therapies and provide new options for treating resistant fungal strains. This strategy could also help reduce the side effects of traditional antifungals, such as gastrointestinal upset and skin reactions.
Fungal infection rates will likely continue to rise. As such, the need for new treatments will only become more urgent. Climate change is already causing new human pathogenic fungi to emerge and spread, including Candida auris. C. auris is often resistant to treatment and spread rapidly in health care facilities during the COVID-19 pandemic. According to the Centers for Disease Control and Prevention, strained health care systems, overuse of immunosuppressants and misuse of antibiotics have all been implicated in outbreaks of C. auris.
In the future, researchers could use artificial intelligence to create better antifungal molecular machines. By using AI to predict how different molecular machines will interact with fungi and human cells, we can develop safer and more effective antifungal molecules that specifically kill fungi without harming healthy cells.
Antifungal molecular machines are still in the early stages of development and are not yet available for routine clinical use. However, continuing research gives hope that these machines could one day provide better treatments for fungal infections and other infectious diseases.
Provided by
The Conversation
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Source: Read Full Article