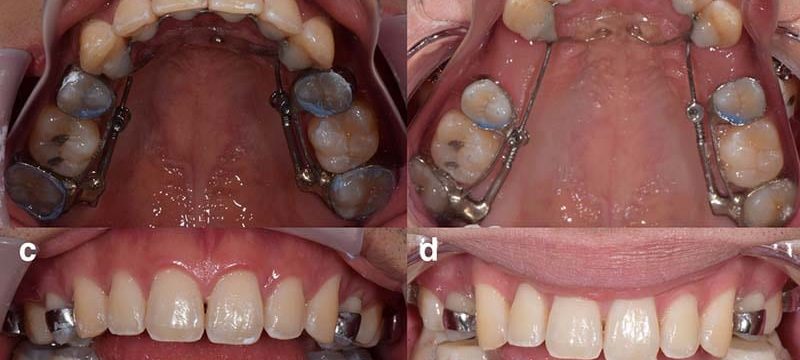
Boja Kragulj, an accomplished clarinetist who once performed with orchestras in New York, Philadelphia, and Jacksonville, Florida, has already lost four teeth. And she expects to lose at least a dozen more.

Clarinetist Boja Kragulj says she used the AGGA (Anterior Growth Guidance Appliance) in hopes of correcting her misaligned bite and improving her breathing without surgery.
Five years ago, seeking to correct her bite and improve her breathing, Kragulj tried a dental device that she was told would put pressure on her upper palate, lengthening her jawbone to fix her issues without surgery, according to an ongoing lawsuit she has filed in federal court. Kragulj said she discovered the device through Facebook, and it sounded “miraculous.”
What she said happened next was ghastly. Kragulj alleged in her lawsuit that instead of changing her jaw, the device pushed her teeth forward through the bone that anchors their roots in place, which put her front teeth in jeopardy. Dozens of photos provided by her attorney show that over time her teeth bulged out of her mouth, warping her smile into a twisted mess. In the three years since filing her suit, Kragulj has had four unsalvageable teeth removed and two others ground to nubs, she said.
Now Kragulj’s only option is to undergo far more extensive surgeries than she faced before, according to her lawsuit. She described pain when eating anything that must be chewed and sometimes struggles to speak clearly through false teeth. And her livelihood is lost: Despite decades of training, Kragulj recently said she can no longer play clarinet well enough to perform or teach.
More than 10,000 dental patients have been fitted with an Anterior Growth Guidance Appliance, or “AGGA,” according to court records. But the unproven and unregulated dental device, often costing patients about $7,000, has not been evaluated by the FDA, according to a months-long joint investigation by KHN and CBS News. The FDA relies on device companies to submit new products for evaluation, and because the AGGA was never submitted, it has been sold to patients without that government review.
“They’re still selling it. And still teaching classes. And still putting it in people’s mouths,” Kragulj, 42, said in an interview.
Dentists across the country promote the AGGA on their websites, often claiming it can “grow,” “remodel,” or “expand” an adult’s jaw without surgery, sometimes saying it has the potential to make patients more attractive and treat common ailments like sleep apnea and TMJ. However, after reviewing dental scans that the AGGA inventor submitted in court to prove the device works, eight experts told KHN and CBS News the scans show signs of the AGGA displacing teeth instead of expanding the jaw. Some experts said, based on their experience with former AGGA patients, the device caused tens of thousands of dollars in damage to the patient’s mouths.
Dr. Marianna Evans, a Philadelphia orthodontist and periodontist who has examined multiple AGGA patients experiencing pain or complications, said she was reminded of gruesome, decades-old experiments that intentionally displaced the teeth of monkeys and dogs to test the limits of orthodontia.
“These studies could not be done on humans because it was ethically wrong,” Evans said. “So now something I had only seen in very old studies that were published in black and white, on animals, I saw in my patients with 3D X-rays.”
At least 20 AGGA patients, including Kragulj, have in the past three years filed lawsuits detailing their complaints about the device, claiming it left them with flared teeth, damaged gums, exposed roots, or erosion of the bone that holds teeth in place. Some plaintiffs said in lawsuits they would lose teeth and added in interviews that they no longer have enough healthy bone to replace their teeth with dental implants.
Most of their lawsuits do not name the dentists who installed the device as defendants, but are filed against the AGGA’s inventor, its manufacturer, and companies that train dentists to use it, alleging they profit from false claims about a device that does not — and cannot — work.
All the AGGA lawsuits are ongoing. Attorneys for the inventor, Dr. Steve Galella, and the company he leads, the Facial Beauty Institute, have in court filings denied liability and argued that plaintiffs were appropriately warned of potential complications from the device, including “teeth dying” or “removal of teeth.” The Las Vegas Institute, which previously held AGGA classes for dentists and promoted the device on Facebook, denied liability in court and has a pending motion to end claims in one lawsuit in which it is named as a defendant. And the AGGA’s manufacturer, Johns Dental Laboratories, has settled one lawsuit for an undisclosed amount but continues to fight allegations in the rest of the cases.
Galella, 70, a Tennessee dentist who invented the AGGA in the 1990s, declined to be interviewed after being contacted by phone, email, and in person. His attorney, Alan Fumuso, said in a written statement that Galella “had not been made aware of any complaints” about the device prior to the recent lawsuits.
“The [AGGA], when properly used, is safe and can achieve beneficial results for the patient,” Fumuso said. “This is not only the personal observation and experience of Dr. Galella, but also the experience of other dentists as well.”
The plaintiffs do not allege in their lawsuits that Galella treated them but that he or his company consulted with each of their dentists about their AGGA treatment.
For this article, KHN and CBS News journalists interviewed 11 dental patients who said they were harmed by the AGGA — eight of whom have active lawsuits concerning the device — plus attorneys who said they represent or have represented at least 23 others. In every case, the patients said in lawsuits or interviews that they were convinced the device would expand their jaws or improve their breathing and mistakenly assumed the AGGA would not be for sale unless it was proven safe and effective.
None of their jawbones expanded, the patients alleged in lawsuits and interviews.
Leigh Peterson, 47, of Ohio, spent $7,000 on AGGA treatment in hopes of alleviating her TMJ, or temporomandibular joint disorder, which had caused her pain since she was a teen.
Within months of AGGA use, Peterson said, her teeth were so loose she could feel them move when rubbing moisturizer on her cheeks. Kissing her boyfriend became uncomfortable.
Peterson, who does not have an active lawsuit, said that according to a dental specialist she will need at least one round of bone grafts to stabilize her teeth.
“I feel like all I have to look forward to now is treatment and pain and fear and debt,” Peterson said. “And I just regret it. I wish I’d never done any of this.”
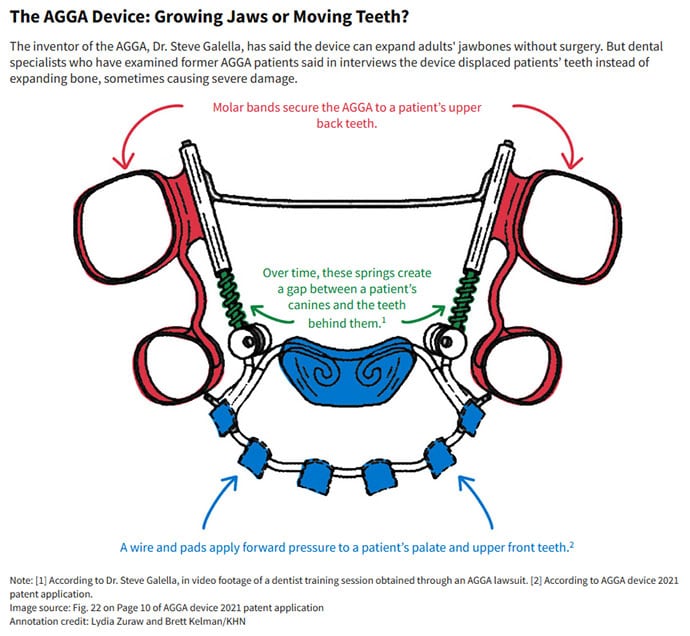
The AGGA, which was recently rebranded as the Osseo-Restoration Appliance, resembles a retainer and uses springs to apply pressure to the front teeth and upper palate, according to a patent application filed in 2021. The version of AGGA intended for adults is affixed to a patient’s molars, typically worn for several months, and must be removed by a medical professional. Galella said pressure from the device causes an adult’s jaw to “remodel” forward “to where the body really wants it to be,” according to video footage from one of his dentist trainings produced in discovery in an AGGA lawsuit. In the video, Galella describes this transformation as the key to “curing” patients and making them more beautiful.
“We fix the facial biology,” Galella said in the video.
However, in a series of interviews with orthodontists, periodontists, and maxillofacial surgeons — all of whom have more training than the average dentist — these experts said that while it is possible to expand the jaws of children without surgery, jawbones stop growing forward as people mature into adulthood. Experts who have examined patients fitted with an AGGA said the device aggressively moved teeth, sometimes creating an illusion of jaw growth by tilting some teeth forward and forcing gaps between others. In the worst cases, those experts have seen teeth shoved so far out of position that their roots are pushed free of the bone and into the gums.
Dr. Kasey Li, a California maxillofacial surgeon and sleep apnea specialist, last year published a study — the abstract of which appeared on a National Institutes of Health website — describing loose teeth and bone loss among AGGA patients he has examined.
In an interview, Li described the AGGA device as “medieval” and said using it to try to expand a jaw is not unlike trying to make your house bigger by simply pushing on the wooden framing in the walls.
“The entire concept of this device, of this treatment, makes zero sense,” Li said. “It doesn’t grow the jaw. It doesn’t widen the jaw. It just pushes the teeth out of their original position.”
Rolling Out the AGGA
The AGGA device has been used on patients for about 15 years. Its biggest promoter is Galella, who operates out of a small, unremarkable clinic in a strip mall in the Memphis suburbs.
Galella said in a 2021 sworn deposition in one of the lawsuits that he has applied the AGGA to about 600 patients and prepared treatment plans for patients getting an AGGA from another dentist on about 9,800 occasions, collecting a “royalty” of $50 to $65 each time the device is made.
The Facial Beauty Institute has also taught an undisclosed number of dentists to use the device during three-day courses costing about $5,000, according to the company’s website. The Las Vegas Institute, also known as LVI Global, offered similar AGGA classes for years and lists on its website about 75 dentists across the U.S. and Canada who have taken that class.
Dave Hornblower, 36, of Ontario, who was fitted with an AGGA in 2019 by a dentist who trained at the Las Vegas Institute, now expects to lose multiple teeth, according to his lawsuit against the company, the inventor, and other defendants.
Hornblower said in an interview the AGGA did not improve his breathing and he now feels pain whenever he makes a “TH” sound, brushing his tongue against the back of his front teeth.
“My dentist said he’d went to courses, seen the evidence, and he seemed very sure of himself, so I was sure of him,” Hornblower said. “He told me it would do all that magical stuff, and I believed him.”
William Schuller, an attorney for the Las Vegas Institute, said in a phone interview that the Institute disputes claims the AGGA is “inherently dangerous” or “has no utility to adults.” Schuller said AGGA training is no longer offered at the institute and disputed that the institute ever taught dentists to use the device.
“I wouldn’t go so far as to say that LVI directly taught dentists to use the AGGA device ever,” he said. “LVI is a campus that provides the ability to teach courses in a variety of procedures and techniques. The doctors who taught the courses were associated with Dr. Galella. It was his course and his course materials that he prepared.”
However, according to a sworn deposition filed in one of the AGGA lawsuits, the AGGA training at the Las Vegas Institute was for years taught by the company’s “co-orthodontic directors,” Dr. David Buck and Dr. Timothy Gross. Buck said in that deposition he created lectures and wrote materials for the course, which were approved by Galella and the leadership of the institute, which kept 70% of the tuition paid for the trainings. A slideshow presentation from one of these training sessions, filed as an exhibit in another AGGA lawsuit, identifies Buck and Gross as “clinical instructor[s]” at the institute.
When pressed during his deposition, Galella said he was not aware of any peer-reviewed studies or clinical trials demonstrating that the AGGA works as claimed on patients whose jaws have finished growing. Galella said his confidence in the device comes from years of using it on patients and dental scans he had not published.
“It proved it to me,” Galella said. “But for the rest of the world, I hadn’t posted anything. Sorry.”
Galella’s company has posted a non-peer-reviewed “white paper” that summarizes the theory behind the AGGA and contains one image from a dental scan of an unidentified, presumably adult patient that describes 1 to 3 millimeters of “outward relocation” of the upper jawbone after wearing an AGGA for four months. The paper says research on the AGGA “takes some time” and “we have begun this research in earnest.”
Schuller acknowledged the lack of peer-reviewed evidence behind the AGGA.
“We are not aware of any peer-reviewed articles regarding AGGA working,” he said in an interview, “and we’re not aware of any peer-reviewed articles regarding AGGA not working. So my understanding is that there is no literature to speak to it one way or the other.”
Faced with the lack of professional studies, a federal judge last year ordered Galella to turn over a sample of his dental scans. Galella was required to provide the plaintiffs with before-and-after scans from five patients over age 30 that demonstrate the AGGA is effective.
Those scans offer no proof, according to an expert witness enlisted by the plaintiffs. In a sworn affidavit filed with the court, Dr. Ricky Harrell, who leads the residency program at the Georgia School of Orthodontics, said Galella’s dental scans “demonstrate no appreciable growth” of the adult jawbones.
KHN and CBS News had those scans reviewed by eight independent experts, including orthodontists, periodontists, maxillofacial surgeons, and faculty from dental colleges at Columbia, Harvard, and the University of Florida. None of these experts was involved in any of the AGGA lawsuits at the time of their interviews.
Every expert had the same response: Galella’s scans showed patients’ teeth had moved but their jaws remained unchanged.
“It is proof,” said Dr. Richard Roblee, an Arkansas orthodontist who reviewed the scans. “Proof that the [AGGA] is not working correctly, not doing what they say. That is the proof that he has given.”
Roblee said he has examined at least 15 people he said were harmed by the AGGA and has never seen another dental technique cause “this much damage” to so many patients.
Dr. George Mandelaris, a Chicago-area periodontist and member of the American Academy of Periodontology Board of Trustees, said Galella’s dental scans show “harm” to the bone that holds teeth in place. Mandelaris said he has consulted with 11 AGGA patients, including Kragulj, who looked as if “a bomb went off in her mouth.”
Dr. Sercan Akyalcin, the head of orthodontics at Harvard, said the scans showed the patients’ upper frontmost teeth were pushed forward but did not show that their jawbones expanded.
Dr. Millie Embree, a professor of orthodontics at Columbia, and Dr. Anita Gohel, the head of oral radiology at the University of Florida, each said they saw that patients were losing teeth in the scans that Galella chose to validate the AGGA’s effectiveness.
“I’m a little surprised that this was the best evidence,” Gohel said. “I wonder what the rest is.”
The AGGA appears to be off the radar of the FDA, which is responsible for regulating medical and dental devices in the United States. Device manufacturers are supposed to register new products with the agency, and any devices that pose even a moderate risk to a patient can be required to go through a pre-market review to check if they are safe and effective.
In an emailed statement, the FDA confirmed it had no record of the AGGA being registered in its device database but would not comment on whether the device should have been registered or if it would be investigated. The agency would not say if it was aware of the AGGA prior to being contacted by KHN and CBS News.
The AGGA’s exclusive manufacturer, Johns Dental Laboratories, located in Indiana, said in a court document it has no record of communicating with the FDA about the AGGA before beginning to make or sell it. Johns Dental said in court the AGGA falls into the FDA’s least-risky classification for devices, similar to a dental retainer, and is exempt from a pre-market clearance under a statutory exemption for dental labs. Johns Dental attorney Jeffrey Oberlies declined to comment.
Galella said in his deposition that he believed the AGGA was outside of the FDA’s jurisdiction.
Cara Tenenbaum, a former senior policy adviser in the FDA’s device center, said the AGGA is within FDA jurisdiction and it was “incredibly problematic” that it was not registered, at least in part because that is how the FDA collects reports of negative effects.
If properly registered, Tenenbaum said, the AGGA might be classified with devices that reposition the jaw or prevent snoring, which are in a more tightly regulated category than what Johns Dental cited in court. Tenenbaum said the FDA was most likely unaware of the AGGA and she suspects it will investigate once alerted to allegations of patient harm.
Scott Charnas, a New York attorney who represents numerous AGGA patients, said he believed a more proactive FDA would have discovered and investigated the device years ago.
“It’s just going to go on and on unless someone does something about it,” Charnas said. “Somebody needs to step up.”
AGGA Inventor: “It’s OK to Make a Crapload of Money”
Both Galella and the Las Vegas Institute have said in dentist trainings that the AGGA can “cure” TMJ and sleep apnea, according to the AGGA training video footage and a slideshow presentation obtained from the ongoing lawsuits.
Galella is heard in the video telling dentists that customers are “gonna beat your door down” because the AGGA can cure patients instead of merely treating their symptoms. He says some patients who want to lessen their pain or improve their looks will “pay anything — anything! — to have that problem resolved.”
“It’s OK to make a crapload of money,” Galella tells dentists in the video. “You’re not ripping anybody off. You’re curing them. You’re helping them. You’re making their life totally beautiful forever and ever.”
Beauty was also a focal point of AGGA classes at the Las Vegas Institute, where the slideshow presentation features photos of celebrities and models. Images of decorated Olympic swimmer Michael Phelps and Princess of Wales Kate Middleton are shown as examples of the kind of unattractive, “underdeveloped midface” that the AGGA is claimed to correct. Middleton is specifically described as “remarkably not” beautiful.
“Why are we here?” reads the first page of the slideshow. “To treat ugly faces.”
The Las Vegas Institute frequently promoted the AGGA in a Facebook group where many of the patients interviewed for this story say they were first persuaded to use the device. The group outwardly appears to be a discussion space for people with jaw problems, and membership is controlled by the Las Vegas Institute.
“We were looking for this holy grail type of deal,” said Karan Gill, who wore an AGGA for months and alleges in a lawsuit his teeth were left loose and sensitive. “The people who were promoting the AGGA in these Facebook groups and such — that’s the way they were talking about it.”
Five former members described the Facebook group as a pro-AGGA echo chamber, where anyone who asked for proof that the AGGA works was hushed or banned.
“If you seek the truth outside, you will be excommunicated,” said Nick Hamilton, 40, a former member of the Facebook group who has an ongoing lawsuit against Galella. “I was asking too many questions. And I started talking openly with other people that were having issues. And they kicked us all out.”
A KHN-CBS News review of the Facebook group postings the past six years uncovered at least five posts in which Las Vegas Institute CEO Bill Dickerson said that the AGGA is “growing bone” or can “grow the maxilla,” which is another name for the upper jaw.
“[I]nstead of just moving teeth … we are growing bone … it’s awesome,” Dickerson wrote in a 2017 post about the AGGA in the Facebook group.
Dickerson has since changed his view. Last year, in a sworn deposition filed in an ongoing lawsuit, Dickerson said he did not believe the AGGA could grow the maxilla and agreed it would be misleading to say it could. Dickerson said in the deposition he began to question the claims of what the AGGA could do in 2020, and after reviewing some patients’ dental scans, he severed ties with Galella and the Facial Beauty Institute.
Kragulj, the clarinetist from the beginning of this article, said she discovered the AGGA through a Facebook video from Galella’s Facial Beauty Institute. According to her lawsuit, she got a device in 2018 and wore it for about 14 months, by which point she had sustained “irreversible” damage to the bone that holds her teeth in place.
Eventually, Kragulj sought help from the man who knew the AGGA better than anyone: Galella. She said she traveled to his Facial Beauty Institute for a consultation, expecting an elite academic facility but finding only a small clinic with aging wallpaper and broken equipment.
Kragulj said Galella looked in her mouth and, after an audible sigh, offered to fix her for $15,000 — plus as much as $15,000 more per tooth. Galella confirmed that meeting and approximate cost in his deposition.
After the meeting, Kragulj decided she was done with Galella, according to her lawsuit. She said she returned to the traditional surgeons and specialists she once eschewed, and the first orthodontist she saw described her teeth as “the worst thing he’d ever seen.”
“They were hanging on by a thread, and the bone was gone,” Kragulj said in an interview. “So it was an extravagant process to get to a place where I could even have fake teeth.”
Kragulj said that since abandoning the AGGA treatment she had to remove four front teeth and was fitted with a dental bridge of false teeth. She said she will need surgery to fix the underlying problems in her jaw and will likely need to replace her upper teeth with prosthetics.
Her entire treatment will cost, by her estimate, a minimum of $150,000, followed by a lifetime of maintaining and replacing dental implants, she said.
Kragulj said it is unlikely she will ever play the clarinet professionally again and as of now she cannot play properly for even a minute without pain.
“My inner world is very silent,” Kragulj said. “It was my voice.”
CBS News producer Nicole Keller contributed to this article.
Source: Read Full Article