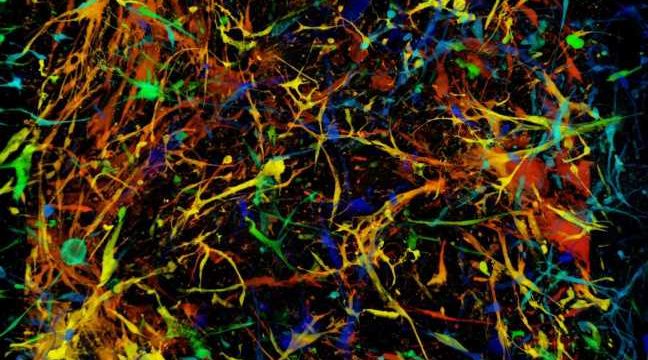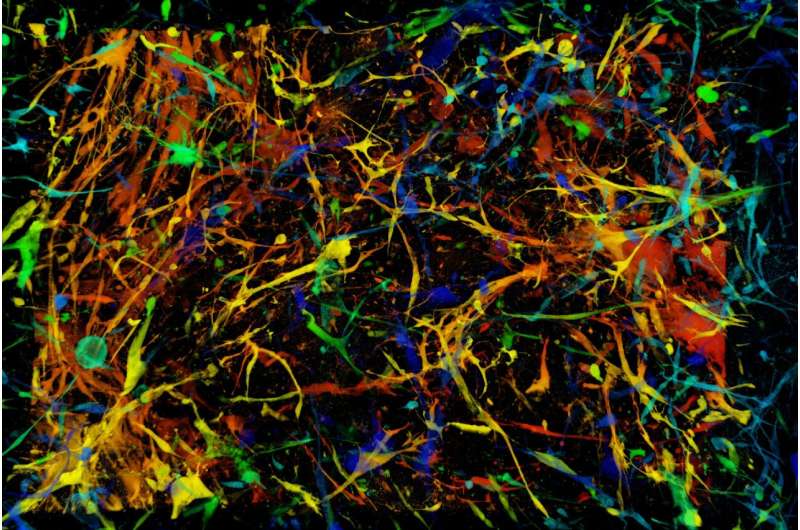
Glioblastomas are the most common malignant tumors of the adult brain. They resist conventional treatment, including surgery, followed by radiation therapy and chemotherapy. Despite this armamentarium, glioblastomas inexorably recur.
In a new study published in Nature Communications, Isabelle Le Roux (CNRS) and her colleagues from the “Genetics and development of brain tumors” team at Paris Brain Institute have shown that the elimination of senescent cells, i.e., cells that have stopped dividing, can modify the tumor ecosystem and slow its progression. These results open up new avenues for treatment.
Glioblastoma, the most common adult brain cancer, affects 2 to 5 in 100,000 individuals. While the incidence of the disease is highest in those between 55 and 85 years old, it is increasing in all age groups. This effect can’t be attributed to improved diagnostic techniques alone, suggesting the influence of environmental factors hitherto unidentified.
People with the disease have a median survival of 15 months after diagnosis, as the tumor infiltrates the brain very quickly. “There is an urgent need to better understand the biology of the tumor, including the diversity of cell types of which it is composed, and their role,” Le Roux explains. “The challenge is to find new therapeutic targets and significantly increase the lifespan of patients.”
Finding the weak spot of glioblastoma is no easy task. One recent approach consists in targeting a key biological process: cellular senescence. Initially identified during the normal aging of cells, it corresponds to the loss of their ability to divide. Interruption of the cell cycle has an advantage: it prevents the uncontrolled division of malignant cells. In that case, senescence contributes to the body’s anti-tumor response.
“Long considered a simple marker of aging, we now know that senescence occurs throughout life, especially in response to genotoxic stress—that is, an event that disrupts or damages DNA, such as chemotherapy,” says Alexa Saliou, a doctoral student in the team and co-first author of the article.
When cells enter senescence, they secrete various molecules. This is called the senescence-associated secretory phenotype—or secretome. “The secretome can influence the cellular environment in a beneficial or detrimental way. For example, it can activate the immune system or, conversely, induce the formation of blood vessels that contribute to the irrigation of the cancerous tissue,” adds the researcher. “It all depends on the molecules secreted.”
Although the effects of senescence may seem paradoxical at first sight, recent studies show that it is all a question of temporality… and context. “In the short term, the secretome is involved in recruiting immune cells to eliminate tumor cells,” Le Roux explains. “But in the long term, the accumulation of senescent cells can promote the destruction of the extracellular matrix—which allows the organization of cells into tissue—and the proliferation of malignant cells.”
The researchers wondered whether there was senescence in glioblastoma and, if so, what role it might play in the cancer progression. To do this, they investigated both an animal model of glioblastoma and tumor tissue removed from patients during surgery.
The team first examined 28 patient tumors. They found, in varying proportions (0.4% to 7% of the original mass of glioblastoma), senescent cells of different cell types—tumoral, immune, or glial—located mainly in areas of malignant cell proliferation, as well as in necrosis zones.
In mice, suppressing a part of the senescent tumor cells made it possible to modify the immune activity within the tumor and extend the animal’s lifespan. The researchers then defined a characteristic signature of senescence based on the expression of 31 genes in mice and ensured that it was identical in humans. “We observed that the strong expression of this signature was associated with a poor prognosis,” adds Alexa Saliou. “This shows the pro-tumor action of senescence in glioblastoma.”
Modulating cellular senescence could therefore constitute a new therapeutic avenue to be combined with conventional treatments—to increase their effectiveness. “Eventually, we could consider treating patients with senolytics, i.e., molecules that target senescent cells to destroy them,” says the researcher.
“In the near future, we hope to see the emergence of new senolytics capable of crossing the blood-brain barrier—which separates the brain from the general bloodstream. This is the big challenge today, as few therapeutic molecules are able to enter the brain. They will also need to cause few side effects if they are to be integrated into patients’ treatments. There is still a long way to go.”
More information:
Rana Salam et al, Cellular senescence in malignant cells promotes tumor progression in mouse and patient Glioblastoma, Nature Communications (2023). DOI: 10.1038/s41467-023-36124-9
Journal information:
Nature Communications
Source: Read Full Article