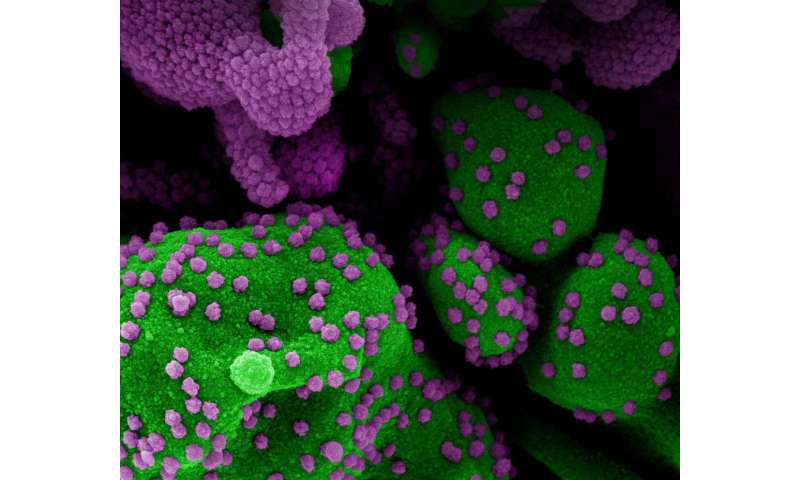
More than 300 scientists and clinicians from the federal government, industry and academia published a report of their conclusions and recommendations on COVID-19 serology studies online in Immunity. The group gathered for an online workshop in May to discuss the role of serology testing in understanding and responding to the COVID-19 public health crisis and to explore strategies to address key scientific knowledge opportunities and gaps in the emerging field. Serology tests for COVID-19 are designed to detect antibodies against SARS-CoV-2, the virus that causes COVID-19. While such tests do not diagnose active infection, they can indicate prior infection with SARS-CoV-2 that may have been missed because a person did not experience significant symptoms or access testing while infected.
The COVID-19 Serology Studies workshop was convened by an interagency working group comprised of experts from the U.S. Department of Health and Human Services—including scientists at the National Institute of Allergy and Infectious Diseases (NIAID), the National Cancer Institute (NCI), and the National Heart, Lung and Blood Institute (NHLBI), parts of the National Institutes of Health, as well as the Centers for Disease Control and Prevention and the Biomedical Advanced Research and Development Authority—and the Department of Defense. Attendees assessed efforts to better understand the implications of serology test results, to produce and validate test kits, and to quantify undetected cases of SARS-CoV-2 infection.
Source: Read Full Article
