The human organism requires a variety of small molecules, such as sugars or fats, in order to function properly. The composition of these so-called metabolites and their interaction—the metabolism—varies from person to person and is dependent not only on external influences, such as nutrition, but also to a significant extent on natural variations in our genetic make-up. In an international study, scientists from the Berlin Institute of Health (BIH) and Charité-Universitätsmedizin Berlin joined forces with colleagues from the United Kingdom, Australia and the United States and discovered hundreds of previously unknown variations in genes that have a sometimes drastic impact on the concentration of these small molecules in the blood. The researchers have now published their findings in the journal Nature Genetics.
The concentration and composition of metabolites—small molecules in the blood or tissue fluid—provide information about biological processes in the human body. They therefore serve as important biomarkers in clinical medicine, for example in the diagnosis of diseases or in checking the effectiveness of a therapy. Interestingly, the composition of metabolites differs from person to person, independent of external influences such as illness or diet. This is because the blueprints for the proteins that influence metabolite concentration, such as enzymes and transporter proteins, also differ between individuals. Often, the tiniest genetic variants can cause a metabolic enzyme to be more or less active or a transporter protein to be more or less efficient, thus raising or lowering the concentration of metabolites.
Data analyzed from 85,000 people
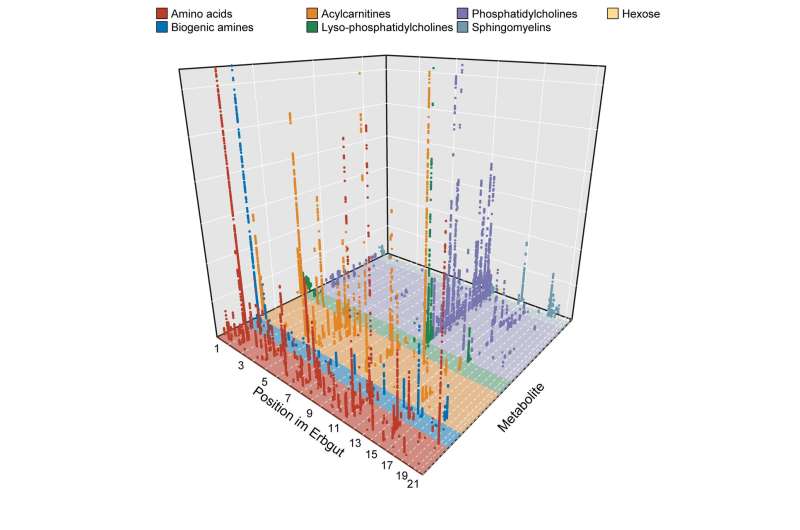
The team led by Claudia Langenberg, BIH Professor of Computational Medicine, has now investigated the effect of genetic variants on 174 different metabolites. “We found a surprising number of correlations between certain genetic variants and changes in the concentration of small molecules in the blood,” reports the epidemiologist. “In most cases, the genetic variants cause changes in the blueprint of key metabolism regulators, like enzymes or transporters.”
To explore these correlations, Langenberg’s team needed huge amounts of data. “For our studies, we used large databases that gave us the blood test results and genetic information of a total of around 85,000 people,” explains Maik Pietzner, lead author of the study and a scientist in Langenberg’s laboratory. “In doing so, we were able to successfully demonstrate that it is possible to jointly evaluate data from a variety of small individual studies, even across technological boundaries.”
Genetic variants can contribute to common diseases
The scientists’ work is highly relevant to medicine, because it can explain how naturally occurring genetic variants that influence the metabolism contribute to the onset of common diseases, such as diabetes mellitus, as well as rare diseases. For example, high levels of the amino acid serine in the blood seem to provide protection against a rare eye disease called macular telangiectasia—knowledge that opens up new therapeutic avenues. In another study, the authors were also able to show that an individual’s genetic risk for altered serine metabolism can aid in the early diagnosis of this serious eye disease. They have also identified a new mechanism that explains how the disrupted transmission of signals via the GLP-2 receptor increases the risk of developing type 2 diabetes.
“What was special about our study were the extreme effects that we observed and their potential relevance for medical research,” explains Langenberg. “For example, we were able to detect genetic variants that have an influence on metabolism a good three times as strong as the already known effects of more common genetic variations, for example on body mass index.”
Source: Read Full Article
